Cancer Res Treat.
2006 Jun;38(3):159-167.
The Synergism between Belotecan and Cisplatin in Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Korea. bangyj@plaza.snu.ac.kr
- 3Cancer Research Institute (CRI), Seoul National University College of Medicine, Seoul, Korea.
- 4National Cancer Center, Goyang, Korea.
Abstract
-
PURPOSE: We wanted to demonstrate the anti-cancer effect and interaction between belotecan and cisplatin on gastric cancer cell line and we evaluated the mechanisms of this synergistic effect in vitro.
MATERIALS AND METHODS
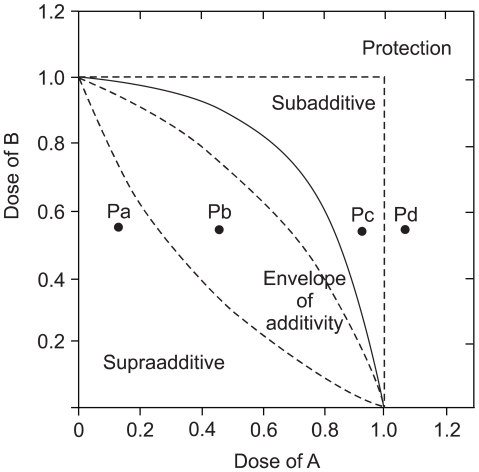
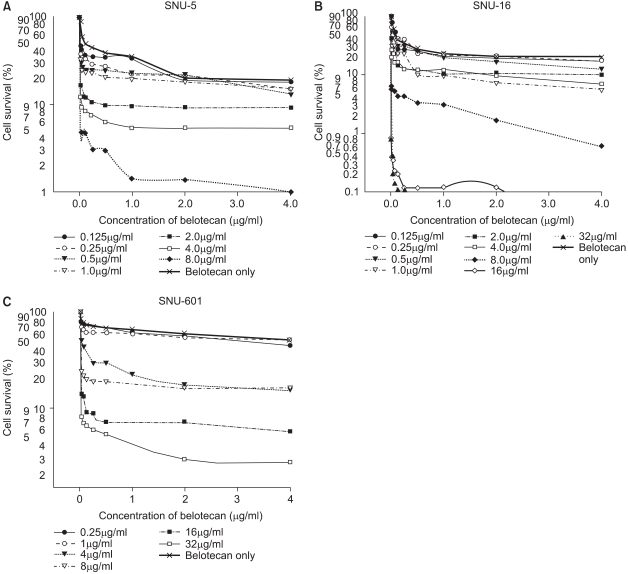
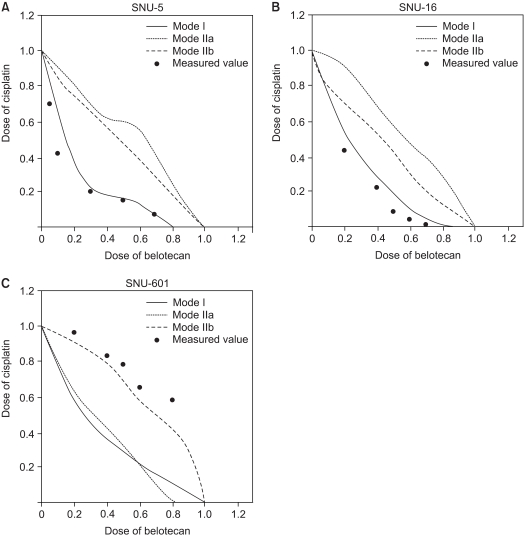
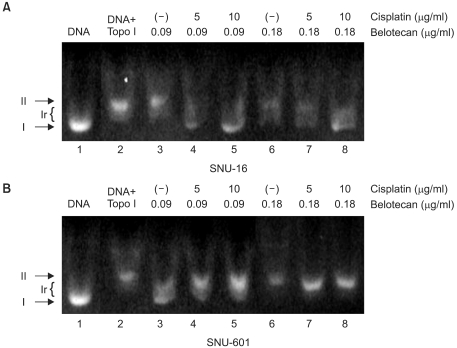
The growth inhibitory effect of belotocan and cisplatin against several gastric cancer cell lines (SNU-5, SNU-16 and SNU-601) was estimated by tetrazolium dye assay. The effect of a combination treatment was evaluated by the isobologram method. The biochemical mechanisms for the interaction between the drugs were analyzed by measuring the formation of DNA interstrand cross-links (ICLs) and DNA topo-I activity.
RESULTS
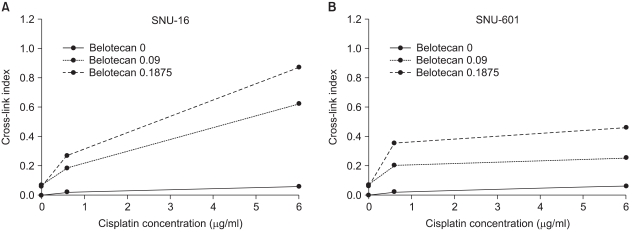
Belotecan showed synergism with cisplatin for growth inhibitory effect on the gastric cancer cell lines SNU-5, and SNU-16, but this was subadditive on the SNU-601 cell line. The formation of DNA ICLs in SNU-16 cells by cisplatin was increased by combination with belotecan, but this was not affected in SNU-601 cells. The topo-I inhibition by belotecan was enhanced at high concentrations of cisplatin in SNU-16, but not in SNU-601 cells.
CONCLUSION
Belotecan and cisplatin show various combination effect against gastric cancer cells. The synergism between cisplatin and belotecan could be the result of one of the following mechanisms: the modulating effect of belotecan on the repair of cisplatin-induced DNA adducts and the enhancing effect of cisplatin on the belotecan-induced topo-I inhibitory effect.
Keyword
Figure
Reference
-
1. Bae JM, Won YJ, Jung KW, Park JG. Annual report of the Central Cancer Registry in Korea-2000: based on registered data from 131 hospitals. Cancer Res Treat. 2002; 34:77–83.2. Rowinsky EK, Kaufmann SH. Topotecan in combination chemotherapy. Semin Oncol. 1997; 24(6):Suppl 20. S20-11–S20-26.3. Ohno R, Okada K, Masaoka T, Kuramoto A, Arima T, Yoshida Y, et al. An early phase II study of CPT-11: a new derivative of camptothecin for the treatment of leukemia and lymphoma. J Clin Oncol. 1990; 8:1907–1912. PMID: 2230878.
Article4. Masuda N, Fukuoka M, Kusunoki Y, Mtsui K, Takifuji N, Kudoh S, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. 1992; 10:1225–1229. PMID: 1321891.
Article5. Fukuoka M, Niitani H, Suzuki A, Motomiya M, Hasegawa K, Nishiwaki Y, et al. A phase II study of CPT-11, a new derivative of camptothecin for previously untreated non-small cell lung cancer. J Clin Oncol. 1992; 10:16–20. PMID: 1309380.6. Shimada Y, Yoshino M, Wakui A, Nakao I, Futatsuki K, Sakata Y, et al. Phase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancer. J Clin Oncol. 1993; 11:909–913. PMID: 8487053.7. Lee JH, Lee JM, Kim JK, Ahn SK, Lee SJ, Kim MY, et al. Antitumor Activity of 7-[2-(N-Isopropylamino)ethyl]-(20S)-camptothecin, CKD602, as a Potent DNA Topoisomerase I inhibitor. Arch Pharm Res. 1998; 21:581–590. PMID: 9875499.
Article8. Kim HK, Bang YJ, Heo DS, Shin SG, Kim NK. Phase I trial of CKD-602, a novel camptothecin derivative, in patients with advanced solid tumors. Proc Am Soc Clin Oncol. 2002; 21:(abstr 393).9. Song Y, Seo SS, Bang YJ, Kang SB, Nam JH, Ryu SY, et al. Phase II evaluation of CKD-602, a camptothecin analog, administered on a 5-day schedule in patients with recurrent or refractory ovarian cancer. Proc Am Soc Clin Oncol. 2003; 22:(abstr 1877).10. Ma J, Maliepaard M, Nooter K, Boersma AW, Verweij J, Stoter G, et al. Synergistic cytotoxicity of cisplatin and topotecan or SN-38 in a panel of eight solid-tumor cell lines in vitro. Cancer Chemother Pharmacol. 1997; 41(4):307–316. PMID: 9488600.
Article11. Fukuda M, Nishio K, Kanzawa F, Ogasawara H, Ishida T, Arioka H, et al. Synergism between cisplatin and topoisomerase inhibitors, NB-506 and SN-38, in human small cell lung cancer cells. Cancer Res. 1996; 56:789–793. PMID: 8631015.12. Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M, et al. Effects of CPT-11 in combination with other anti-cancer agents in culture. Int J Cancer. 1992; 50:604–610. PMID: 1537625.
Article13. Bungo M, Fujiwara Y, Kasahara K, Nakagawa K, Ohe Y, Sasaki Y, et al. Decreased accumulation as a mechanism of resistance to cis-diamminedichloroplatinum (II) in human non-small cell lung cancer cell lines: relation to DNA damage and repair. Cancer Res. 1990; 50:2549–2553. PMID: 2158392.14. Liu LF, Miller KG. Eukaryotic DNA topoisomerase: two forms of type I DNA topoisomerase from HeLa cell nuclei. Proc Natl Acad Sci USA. 1981; 78:3487–3491. PMID: 6267594.15. Romanelli S, Perego P, Pratesi G, Carenini N, Tortoreto M, Xunino F. In vitro and in vivo interaction between cisplatin and topotecan in ovarian carcinoma systems. Cancer Chemother Pharmacol. 1998; 41:383–390.
Article16. Fichtinger-Schepman AMJ, van der Veer JL, den Hartog JH, Lohman PHM, Reedijk J. Adducts of the antitumor drug cisdiamminedichloroplatinum( II) with DNA: formation, identification, and quantitation. Biochmistry. 1985; 24:707–713.17. Chu G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J Biol Chem. 1994; 269:787–790. PMID: 8288625.
Article18. Swinnen LJ, Ellis NK, Erickson LC. Inhibition of cis-diammine-1,1-cyclobutane dicarboxylatoplatinum(II)-induced DNA interstrand cross-link removal and potentiation of cis-diammine-1,1-cyclobutane dicarboxyl-atoplatinum(II) cytotoxicity by hydroxyurea and 1β-D-arabinofuranosylcytosine. Cancer Res. 1991; 51:1984–1989. PMID: 2009517.19. Esaki T, Nakano S, Tatsumoto T, Kuroki-Migita M, Mitsugi K, Nakamura M, et al. Inhibition by 5-fluorouracil of cis-diamminedichloroplatinum( II)-induced DNA interstrand cross-link removal in a HST-1 human squamous carcinoma cell line. Cancer Res. 1992; 52:6501–6506. PMID: 1423296.20. Eastman A, Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988; 27:4730–4734. PMID: 3167012.
Article21. Parker RJ, Eastman A, Bostick-Bruton F, Reed E. Acquired cisplatin resistance in human ovarian cancer cells is associated with enchanced repair of cisplatin-DNA lesions and reduced drug accumulation. J Clin Invest. 1991; 87:772–777. PMID: 1999494.22. Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y. Structure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relaxation to antitumor activity. Cancer Res. 1989; 49:1465–1469. PMID: 2538227.23. Bellon SF, Coleman JH, Lippard SJ. DNA unwinding produced by site-specific intrastrand cross-links of the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1991; 30:8026–8035. PMID: 1868076.
Article24. van Waardenburg RC, de Jong LA, van Eijndhoven MA, Verseyden C, Pluim D, Jansen LE, et al. Plainated DNA Adducts Enhance Poisoning of DNA Topoisomerase I by Camptothecin. J Biol Chem. 2004; 279:54502–54509. PMID: 15471886.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Belotecan and Cisplatin Combination Chemotherapy for Previously Untreated Extensive-Disease Small Cell Lung Cancer
- Synergistic effect of peroxiredoxin II antisense on cisplatin-induced cell death
- Effect of Topotecan in Combication with Other Antitumor Drugs in Vitro
- Combination chemotherapy with 5-fluorouracil and cisplatin for advanced gastric cancer
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin