Cancer Res Treat.
2006 Dec;38(4):189-197.
Tumor Angiogenesis: Initiation and Targeting : Therapeutic Targeting of an FGF-Binding Protein, an Angiogenic Switch Molecule, and Indicator of Early Stages of Gastrointestinal Adenocarcinomas
- Affiliations
-
- 1Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC. wellstea@georgetown.edu
Abstract
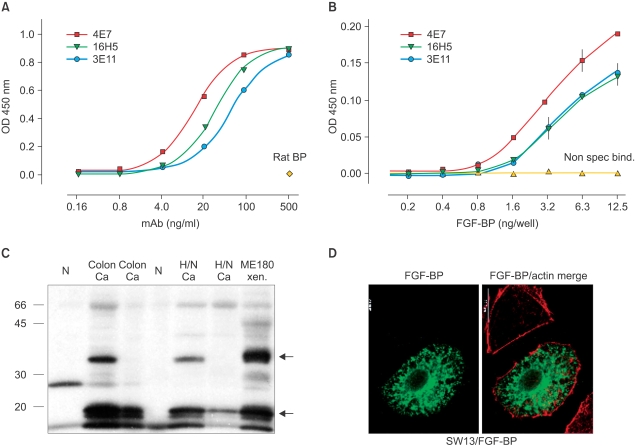
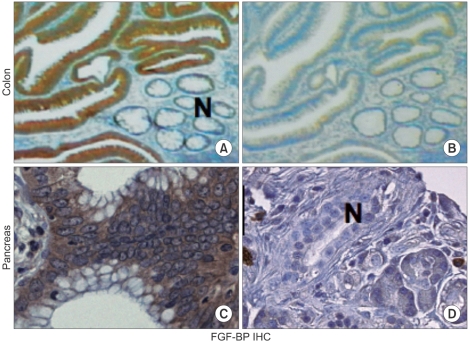
- Tumor angiogenesis has been related to the initiation as well as progression toward more aggressive behavior of human tumors. In particular, the activity of angiogenic factors is crucial for tumor progression. We previously characterized a secreted fibroblast growth factor-binding protein (FGF-BP) as a chaperone molecule, which binds to various FGFs, enhances FGF-mediated biochemical and biologic events and importantly is a crucial rate-limiting factor for tumor-dependent angiogenesis. We generated monoclonal antibodies that target FGF-BP protein and used them as a tool to evaluate frequency and pattern of FGF-BP expression during the malignant progression of pancreas and colorectal carcinoma in archival tissue samples. We found that FGF-BP is dramatically upregulated during the initiation of colorectal and pancreatic adenocarcinoma. Crucial genetic events underlying the initiation and progression of colorectal and pancreatic adenocarcinoma with a particular focus on the modulation of angiogenesis and antiangiogenic therapies are discussed. We propose that the upregulation of the secreted FGF-BP protein during early phases of pancreas and colon cancer could make this protein a possible serum marker indicating the presence of high-risk premalignant lesions. Furthermore, the biological activity of FGF-BP is neutralized by monoclonal antibodies suggesting the potential for antibody-based therapeutic targeting.
Keyword
MeSH Terms
-
Adenocarcinoma*
Angiogenesis Inducing Agents
Angiogenesis Inhibitors
Antibodies, Monoclonal
Biomarkers
Colonic Neoplasms
Colorectal Neoplasms
Fibroblasts
Humans
Pancreas
Pancreatic Neoplasms
Staphylococcal Protein A
Up-Regulation
Angiogenesis Inducing Agents
Angiogenesis Inhibitors
Antibodies, Monoclonal
Staphylococcal Protein A
Figure
Reference
-
2. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000; 6:389–395. PMID: 10742145.
Article3. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996; 86:353–364. PMID: 8756718.
Article4. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002; 29:15–18. PMID: 12516034.
Article5. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990; 82:4–6. PMID: 1688381.
Article6. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285:1182–1186. PMID: 4938153.
Article7. Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972; 175:409–416. PMID: 5077799.8. Risau W. Development and differentiation of endothelium. Kidney Int. 1998; 67(Suppl):S3–S6.
Article9. Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003; 9:685–693. PMID: 12778167.
Article10. Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000; 156:1363–1380. PMID: 10751361.
Article11. Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998; 95:4607–4612. PMID: 9539785.
Article12. Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999; 237:97–132. PMID: 9893348.
Article13. Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999; 103:159–165. PMID: 9916127.
Article14. Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000; 156:361–381. PMID: 10666364.
Article15. Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000; 97:14608–14613. PMID: 11121063.
Article16. Baish JW, Jain RK. Fractals and cancer. Cancer Res. 2000; 60:3683–3688. PMID: 10919633.17. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407:249–257. PMID: 11001068.
Article18. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997; 3:177–182. PMID: 9018236.
Article19. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999; 155:739–752. PMID: 10487832.
Article20. Zhong H, Bowen JP. Antiangiogenesis drug design: multiple pathways targeting tumor vasculature. Curr Med Chem. 2006; 13:849–862. PMID: 16611071.
Article22. Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000; 7:165–197. PMID: 11021964.
Article23. Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004; 20:563–569. PMID: 15475116.
Article24. Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005; 16:159–178. PMID: 15863032.
Article25. Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005; 16:107–137. PMID: 15863029.
Article26. Ray PE, Tassi E, Liu XH, Wellstein A. Role of fibroblast growth factor-binding protein in the pathogenesis of HIV-associated hemolytic uremic syndrome. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R105–R113. PMID: 16352855.
Article27. Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, et al. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001; 276:40247–40253. PMID: 11509569.
Article28. Mongiat M, Otto J, Oldershaw R, Ferrer F, Sato JD, Iozzo RV. Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J Biol Chem. 2001; 276:10263–10271. PMID: 11148217.
Article29. Wu DQ, Kan MK, Sato GH, Okamoto T, Sato JD. Characterization and molecular cloning of a putative binding protein for heparin-binding growth factors. J Biol Chem. 1991; 266:16778–16785. PMID: 1885605.
Article30. Czubayko F, Smith RV, Chung HC, Wellstein A. Tumor growth and angiogenesis induced by a secreted binding protein for fibroblast growth factors. J Biol Chem. 1994; 269:28243–28248. PMID: 7525570.
Article31. Czubayko F, Liaudet-Coopman ED, Aigner A, Tuveson AT, Berchem GJ, Wellstein A. A secreted FGF-binding protein can serve as the angiogenic switch in human cancer. Nat Med. 1997; 3:1137–1140. PMID: 9334727.
Article32. Kurtz A, Wang HL, Darwiche N, Harris V, Wellstein A. Expression of a binding protein for FGF is associated with epithelial development and skin carcinogenesis. Oncogene. 1997; 14:2671–2681. PMID: 9178765.
Article33. Xie B, Tassi E, Swift MR, McDonnell K, Bowden ET, Wang S, et al. Identification of the fibroblast growth factor (FGF)-interacting domain in a secreted FGF-binding protein by phage display. J Biol Chem. 2006; 281:1137–1144. PMID: 16257968.
Article34. McDonnell K, Bowden ET, Cabal-Manzano R, Hoxter B, Riegel AT, Wellstein A. Vascular leakage in chick embryos after expression of a secreted binding protein for fibroblast growth factors. Lab Invest. 2005; 85:747–755. PMID: 15806140.
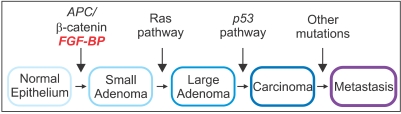
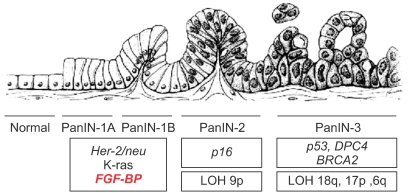
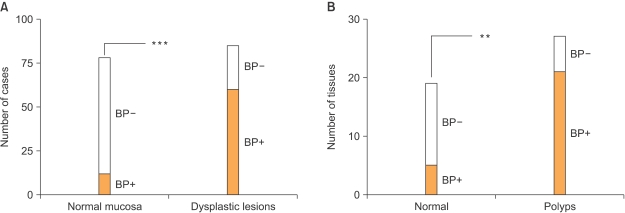
Article35. Kagan BL, Henke RT, Cabal-Manzano R, Stoica GE, Nguyen Q, Wellstein A, et al. Complex regulation of the fibroblast growth factor-binding protein in MDA- MB-468 breast cancer cells by CCAAT/enhancer-binding protein beta. Cancer Res. 2003; 63:1696–1705. PMID: 12670924.36. Ray R, Cabal-Manzano R, Moser AR, Waldman T, Zipper LM, Aigner A, et al. Up-regulation of fibroblast growth factor-binding protein, by beta-catenin during colon carcinogenesis. Cancer Res. 2003; 63:8085–8089. PMID: 14678957.37. Tassi E, Henke RT, Bowden ET, Swift MR, Kodack DP, Kuo AH, et al. Expression of a fibroblast growth factor-binding protein during the development of adenocarcinoma of the pancreas and colon. Cancer Res. 2006; 66:1191–1198. PMID: 16424058.
Article38. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001; 51:15–36. PMID: 11577478.
Article39. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996; 87:159–170. PMID: 8861899.
Article40. Vogelstein B, Kinzler KW. The genetic basis for human cancer. 2001. 2nd ed. Toronto: McGraw-Hill.41. Lamlum H, Ilyas M, Rowan A, Clark S, Johnson V, Bell J, et al. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of the germline mutation: a new facet to Knudson's 'two-hit' hypothesis. Nat Med. 1999; 5:1071–1075. PMID: 10470088.
Article42. Levy DB, Smith KJ, Beazer-Barclay Y, Hamilton SR, Vogelstein B, Kinzler KW. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994; 54:5953–5958. PMID: 7954428.43. Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997; 1332:127–147.
Article44. Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, et al. Association of the APC gene product with beta-catenin. Science. 1993; 262:1731–1734. PMID: 8259518.45. Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993; 262:1734–1737. PMID: 8259519.
Article47. Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995; 92:3046–3050. PMID: 7708772.
Article48. Gumbiner BM. Carcinogenesis: a balance between beta-catenin and APC. Curr Biol. 1997; 7:R443–R446. PMID: 9210368.49. Morin PJ. Beta-catenin signaling and cancer. Bioessays. 1999; 21:1021–1030. PMID: 10580987.50. He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998; 281:1509–1512. PMID: 9727977.51. Aoki M, Hecht A, Kruse U, Kemler R, Vogt PK. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc Natl Acad Sci USA. 1999; 96:139–144. PMID: 9874785.
Article52. Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999; 398:422–426. PMID: 10201372.53. Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990; 247:322–324. PMID: 2296722.
Article54. Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988; 53:549–554. PMID: 2453289.
Article55. Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987; 327:293–297. PMID: 3587348.
Article56. Forrester K, Almoguera C, Han K, Grizzle WE, Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. Nature. 1987; 327:298–303. PMID: 2438556.
Article57. Kressner U, Bjorheim J, Westring S, Wahlberg SS, Pahlman L, Glimelius B, et al. Ki-ras mutations and prognosis in colorectal cancer. Eur J Cancer. 1998; 34:518–521. PMID: 9713302.
Article58. Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989; 49:4682–4689. PMID: 2547513.59. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990; 61:759–767. PMID: 2188735.
Article60. Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 2000; 10:147–154. PMID: 10740269.
Article61. Shibata D, Reale MA, Lavin P, Silverman M, Fearon ER, Steele G, et al. The DCC protein and prognosis in colorectal cancer. N Engl J Med. 1996; 335:1727–1732. PMID: 8929264.
Article62. Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, et al. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics. 1994; 19:525–531. PMID: 8188295.
Article63. Chen YQ, Hsieh JT, Yao F, Fang B, Pong RC, Cipriano SC, et al. Induction of apoptosis and G2/M cell cycle arrest by DCC. Oncogene. 1999; 18:2747–2754. PMID: 10348349.
Article64. Tarafa G, Villanueva A, Farre L, Rodriguez J, Musulen E, Reyes G, et al. DCC and SMAD4 alterations in human colorectal and pancreatic tumor dissemination. Oncogene. 2000; 19:546–555. PMID: 10698524.
Article65. Miyaki M, Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999; 18:3098–3103. PMID: 10340381.
Article66. Koyama M, Ito M, Nagai H, Emi M, Moriyama Y. Inactivation of both alleles of the DPC4/SMAD4 gene in advanced colorectal cancers: identification of seven novel somatic mutations in tumors from Japanese patients. Mutat Res. 1999; 406:71–77. PMID: 10479724.
Article67. Salovaara R, Roth S, Loukola A, Launonen V, Sistonen P, Avizienyte E, et al. Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut. 2002; 51:56–59. PMID: 12077092.
Article68. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectaltumor development. N Engl J Med. 1988; 319:525–532. PMID: 2841597.
Article69. Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989; 244:217–221. PMID: 2649981.
Article70. Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999; 104:263–269. PMID: 10430607.
Article71. Zeng ZS, Sarkis AS, Zhang ZF, Klimstra DS, Charytonowicz E, Guillem JG, et al. p53 nuclear overexpression: an independent predictor of survival in lymph node--positive colorectal cancer patients. J Clin Oncol. 1994; 12:2043–2050. PMID: 7931472.
Article72. Leahy DT, Salman R, Mulcahy H, Sheahan K, O'Donoghue DP, Parfrey NA. Prognostic significance of p53 abnormalities in colorectal carcinoma detected by PCR-SSCP and immunohistochemical analysis. J Pathol. 1996; 180:364–370. PMID: 9014855.
Article73. Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes' B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2003; 21:241–250. PMID: 12525515.
Article74. Garrity MM, Burgart LJ, Mahoney MR, Windschitl HE, Salim M, Wiesenfeld M, et al. Prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with resected Dukes' B2 or C colon cancer: a North Central Cancer Treatment Group Study. J Clin Oncol. 2004; 22:1572–1582. PMID: 15117979.75. Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005; 55:10–30. PMID: 15661684.
Article76. Bodner WR, Hilaris BS, Mastoras DA. Radiation therapy in pancreatic cancer: current practice and future trends. J Clin Gastroenterol. 2000; 30:230–233. PMID: 10777178.77. Berlin JD, Rothenberg M. Chemotherapy for resectable and advanced pancreatic cancer. Oncology. 2001; 15:1241–1249. PMID: 11702956.78. Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001; 25:579–586. PMID: 11342768.79. Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994; 425:357–367. PMID: 7820300.
Article80. Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000; 156:1821–1825. PMID: 10854204.
Article81. Hruban RH, Offerhaus GJ, Kern SE, Goggins M, Wilentz RE, Yeo CJ. Tumor-suppressor genes in pancreatic cancer. J Hepatobiliary Pancreat Surg. 1998; 5:383–391. PMID: 9931387.
Article82. Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer. 2003; 2:8. PMID: 12556241.83. Siddiqi I, Funatomi H, Kobrin MS, Friess H, Buchler MW, Korc M. Increased expression of keratinocyte growth factor in human pancreatic cancer. Biochem Biophys Res Commun. 1995; 215:309–315. PMID: 7575607.
Article84. Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003; 26:344–349. PMID: 12717266.
Article85. Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, et al. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993; 53:5289–5296. PMID: 7693336.86. Kornmann M, Ishiwata T, Beger HG, Korc M. Fibroblast growth factor-5 stimulates mitogenic signaling and is overexpressed in human pancreatic cancer: evidence for autocrine and paracrine actions. Oncogene. 1997; 15:1417–1424. PMID: 9333017.
Article87. Kleeff J, Kothari NH, Friess H, Fan H, Korc M. Adenovirus-mediated transfer of a truncated fibroblast growth factor (FGF) type I receptor blocks FGF-2 signaling in multiple pancreatic cancer cell lines. Pancreas. 2004; 28:25–30. PMID: 14707726.
Article88. Kornmann M, Ishiwata T, Matsuda K, Lopez ME, Fukahi K, Asano G, et al. IIIc isoform of fibroblast growth factor receptor 1 is overexpressed in human pancreatic cancer and enhances tumorigenicity of hamster ductal cells. Gastroenterology. 2002; 123:301–313. PMID: 12105858.
Article89. Kornmann M, Beger HG, Korc M. Role of fibroblast growth factors and their receptors in pancreatic cancer and chronic pancreatitis. Pancreas. 1998; 17:169–175. PMID: 9700949.
Article90. Wagner M, Lopez ME, Cahn M, Korc M. Suppression of fibroblast growth factor receptor signaling inhibits pancreatic cancer growth in vitro and in vivo. Gastroenterology. 1998; 114:798–807. PMID: 9516401.
Article91. Wray CJ, Rilo HL, Ahmad SA. Colon cancer angiogenesis and antiangiogenic therapy. Expert Opin Investig Drugs. 2004; 13:631–641.
Article92. Mancuso A, Sternberg CN. Colorectal cancer and antiangiogenic therapy: what can be expected in clinical practice? Crit Rev Oncol Hematol. 2005; 55:67–81. PMID: 15890525.
Article93. Dirix LY, Vermeulen PB, Hubens G, Benoy I, Martin M, De Pooter C, et al. Serum basic fibroblast growth factor and vascular endothelial growth factor and tumour growth kinetics in advanced colorectal cancer. Ann Oncol. 1996; 7:843–848. PMID: 8922199.
Article94. Landriscina M, Cassano A, Ratto C, Longo R, Ippoliti M, Palazzotti B, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998; 78:765–770. PMID: 9743297.
Article95. Galzie Z, Fernig DG, Smith JA, Poston GJ, Kinsella AR. Invasion of human colorectal carcinoma cells is promoted by endogenous basic fibroblast growth factor. Int J Cancer. 1997; 71:390–395. PMID: 9139874.
Article96. Stoeltzing O, Liu W, Reinmuth N, Parikh A, Ahmad SA, Jung YD, et al. Angiogenesis and antiangiogenic therapy of colon cancer liver metastasis. Ann Surg Oncol. 2003; 10:722–733. PMID: 12900362.97. Tahara E. Meyers RA, editor. Growth factors and oncogenes in gastrointestinal cancers. Encyclopedia of molecular cell biology and molecular medicine. 2005. Weinheim: Wiley-VCH Verlag GmbH & Co KGaA;p. 1–31.
Article98. Akbulut H, Altuntas F, Akbulut KG, Ozturk G, Cindoruk M, Unal E, et al. Prognostic role of serum vascular endothelial growth factor, basic fibroblast growth factor and nitric oxide in patients with colorectal carcinoma. Cytokine. 2002; 20:184–190. PMID: 12543084.
Article99. Herfarth H, Brand K, Rath HC, Rogler G, Scholmerich J, Falk W. Nuclear factor-kappa B activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000; 120:59–65. PMID: 10759764.100. Giavazzi R, Albini A, Bussolino F, DeBraud F, Presta M, Ziche M, et al. The biological basis for antiangiogenic therapy. Eur J Cancer. 2000; 36:1913–1918. PMID: 11000570.
Article101. Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000; 21:505–515. PMID: 10688871.
Article102. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005; 307:58–62. PMID: 15637262.
Article103. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–2342. PMID: 15175435.
Article104. Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005; 23:3697–3705. PMID: 15738537.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RNA Binding Protein as an Emerging Therapeutic Target for Cancer Prevention and Treatment
- Roles of FGF-4 on the Differentiation of Trophoblast Stem (TS) Cells
- Therapeutic Angiogenesis for Cardiovascular Diseases: The Present and Future
- Inhibition Effect of Angiostatin and Endostatin on Human Angiogenesis
- Production of IFN-gamma by TNF-alpha in Macrophages from Tumor Micro Environment; Significance in Angiogenic Switch Control