Cancer Res Treat.
2008 Mar;40(1):36-38.
Hepatitis B Virus Reactivation in a Surface Antigen-negative and Antibody-positive Patient after Rituximab Plus CHOP Chemotherapy
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. kstwoh@korea.ac.kr
- 2Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea.
Abstract
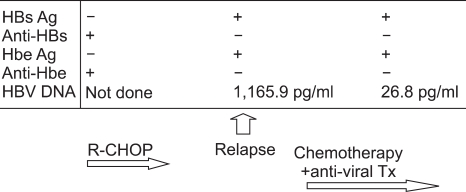
- Rituximab is a monoclonal antibody that targets B-lymphocytes, and it is widely used to treat non-Hodgkin's lymphoma. However, its use has been implicated in HBV reactivation that's related with the immunosuppressive effects of rituximab. Although the majority of reactivations occur in hepatitis B carriers, a few cases of reactivation have been reported in HBsAg negative patients. However, reactivation in an HBsAg negative/ HBsAb positive patient after rituximab treatment has never been reported in Korea. We present here an HBsAg-negative/HBsAb-positive 66-year-old female who displayed reactivation following rituximab plus CHOP chemotherapy for diffuse large B-cell lymphoma. While she was negative for HBsAg at diagnosis, her viral status was changed at the time of relapse as follows: HBsAg positive, HBsAb negative, HBeAg positive, HBeAb negative and an HBV DNA level of 1165 pg/ml. Our observation suggests that we should monitor for HBV reactivation during rituximab treatment when prior HBV infection or occult infection is suspected, and even in the HBsAg negative/HBsAb positive cases.
Keyword
MeSH Terms
-
Aged
Antibodies, Monoclonal, Murine-Derived
B-Lymphocytes
DNA
Female
Hepatitis
Hepatitis B
Hepatitis B e Antigens
Hepatitis B Surface Antigens
Hepatitis B virus
Humans
Korea
Lymphoma
Lymphoma, B-Cell
Lymphoma, Non-Hodgkin
Organothiophosphorus Compounds
Recurrence
Rituximab
Antibodies, Monoclonal, Murine-Derived
DNA
Hepatitis B Surface Antigens
Hepatitis B e Antigens
Organothiophosphorus Compounds
Figure
Reference
-
1. Kumagai K, Takagi T, Nakamura S, Sawada U, Kura Y, Kodama F, et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol. 1997; 8(Suppl 1):107–109. PMID: 9187442.
Article3. Tsutsumi Y, Kanamori H, Mori A, Tanaka J, Asaka M, Imamura M, et al. Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf. 2005; 4:599–608. PMID: 15934864.
Article4. Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001; 344:68–69. PMID: 11187122.
Article5. Yamagata M, Murohisa T, Tsuchida K, Okamoto Y, Tsunoda S, Nakamura M, et al. Fulminant B hepatitis in a surface antigen and hepatitis B DNA-negative patient with diffuse large B-cell lymphoma after CHOP chemotherapy plus rituximab. Leuk Lymphoma. 2007; 48:431–433. PMID: 17325912.
Article6. Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother. 2005; 11:189–191. PMID: 16133710.
Article7. Sera T, Hiasa Y, Michitaka K, Konishi I, Matsuura K, Tokumoto Y, et al. Anti-HBs-positive liver failure due to hepatitis B virus reactivation induced by rituximab. Intern Med. 2006; 45:721–724. PMID: 16819252.
Article8. Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology. 2001; 34:204–206. PMID: 11431752.
Article9. Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely "occult"? Hepatology. 2001; 34:194–203. PMID: 11431751.
Article10. Kim SM, Lee KS, Park CJ, Lee JY, Kim KH, Park JY, et al. Prevalence of occult HBV infection among subjects with normal serum ALT levels in Korea. J Infect. 2007; 54:185–191. PMID: 16564573.
Article11. Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004; 22:927–934. PMID: 14990649.
Article12. Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998; 27:1736–1742. PMID: 9620351.
Article13. Hu KQ. Occult hepatitis B virus infection and its clinical implications. J Viral Hepat. 2002; 9:243–257. PMID: 12081601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hepatitis B Virus Reactivation in a HBsAg-Negative and Anti-HBs-Positive Patient with Diffuse Large B-Cell Lymphoma after Rituximab plus CHOP Chemotherapy
- A Case of Reactivation of Hepatitis B and Fulminant Hepatitis which developed 3 months following Chemotherapy Including Rituximab in a Patient with Lymphoma
- Fulminant Hepatic Failure with Hepatitis B Virus Reactivation after Rituximab Treatment in a Patient with Resolved Hepatitis B
- A Case of Acute Hepatitis B by Occult HBV Infection without HbsAg Seroconversion
- Reactivation of Hepatitis B Virus and Its Prevention in Patients with Rheumatic Diseases Receiving Immunosuppressive Therapy