Cancer Res Treat.
2008 Mar;40(1):11-15.
A Phase II Study of Leucovorin, 5-FU and Docetaxel Combination Chemotherapy in Patients with Inoperable or Postoperative Relapsed Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. sangjael@cau.ac.kr
Abstract
-
PURPOSE: To estimate the effect and toxicity of bimonthly low-dose leucovorin (LV) and fluorouracil (5-FU) bolus plus continuous infusion(LV5FU2) with docetaxel combination chemotheraphy in patients with inoperable or postoperative relapsed gastric cancer.
MATERIALS AND METHODS
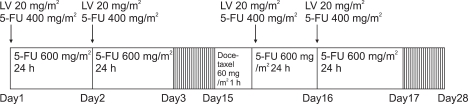
Total 27 patients are enrolled in this study. LV 20 mg/m2 (bolus), 5FU 400 mg/m2 (bolus), 5-FU 600 mg/m2 (24-hour continuous infusion) on day 1, 2, 15, and 16, docetaxel 60 mg/m2 (1-hour infusion) on day 15 every 4 weeks.
RESULTS
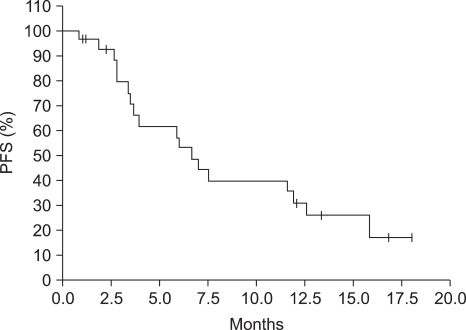
Total of 141 cycles were administered and response rate were 36.8% with 2 complete response (10.5%) and 5 partial response (26.3%) in 19 evaluable patients. The median response duration is 8.1 months (95% CI, 4.0~12.1). The median progression-free survival time is 6.7 months (95% CI, 5.0~8.5) and the median overall survival time is 11.9 months (95% CI, 4.8~19.1). The grade 3-4 toxcity of neutropenia (24.8%) and anemia (11.3%), neutropenic fever (2.8%) is observed. The grade 1 toxcity of injection site reaction is observed all patients and the grade 1-2 toxcity of alopecia is observed 60%.
CONCLUSIONS
LV5FU2 with docetaxel combination chemotheraphy is effective and tolerable in patients with inoperable or postoperative relapsed gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Shin HR, Jung KW, Won YJ, Park JG. 139 KCCR-affiliated Hospital. 2002 Annual Report of the Korea Central Cancer Registry: Based on Registered Data from 139 Hospitals. Cancer Res Treat. 2004; 36:103–114.
Article2. Dupont JB Jr, Lee JR, Burton GR, Cohn I Jr. Adenocarcinoma of the stomach: review of 1,497 cases. Cancer. 1978; 41:941–947. PMID: 638980.
Article3. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006; 24:2903–2909. PMID: 16782930.
Article4. Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer--a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997; 15:368–381. PMID: 8996164.
Article5. Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989; 7:1407–1418. PMID: 2476530.
Article6. Bouche O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol. 2004; 22:4319–4328. PMID: 15514373.7. Sobrero AF, Aschele C, Guglielmi AP, Mori AM, Melioli GG, Rosso R, et al. Synergism and lack of cross-resistance between short-term and continuous exposure to fluorouracil in human colon adenocarcinoma cells. J Natl Cancer Inst. 1993; 85:1937–1944. PMID: 8230285.
Article8. Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005; 23:5660–5667. PMID: 16110025.
Article9. Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996; 13:87–93. PMID: 9013471.
Article10. Ridwelski K, Gebauer T, Fahlke J, Kroning H, Kettner E, Meyer F, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001; 12:47–51. PMID: 11249048.
Article11. Sulkes A, Smyth J, Sessa C, Dirix LY, Vermorken JB, Kaye S, et al. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer. 1994; 70:380–383. PMID: 7914428.12. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.13. Cunningham D, Jost LM, Purkalne G, Oliveira J. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of gastric cancer. Ann Oncol. 2005; 16(Suppl 1):i22–i23. PMID: 15888740.
Article14. Pye JK, Crumplin MK, Charles J, Kerwat R, Foster ME, Biffin A. One-year survey of carcinoma of the oesophagus and stomach in Wales. Br J Surg. 2001; 88:278–285. PMID: 11167881.
Article15. Meta-analysis Group In Cancer. Cancer M-aGI. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998; 16:301–308. PMID: 9440757.16. de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997; 15:808–815. PMID: 9053508.
Article17. Jager E, Heike M, Bernhard H, Klein O, Bernhard G, Lautz D, et al. Weekly high-dose leucovorin versus low-dose leucovorin combined with fluorouracil in advanced colorectal cancer: results of a randomized multicenter trial. Study Group for Palliative Treatment of Metastatic Colorectal Cancer Study Protocol 1. J Clin Oncol. 1996; 14:2274–2279. PMID: 8708717.18. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–4997. PMID: 17075117.
Article19. Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001; 19:2282–2292. PMID: 11304782.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Randomized Phase III Trial of Cisplatin, Epirubicin, Leucovorin, 5-Fluorouracil (PELF) Combination versus 5-fluorouracil Alone as Adjuvant Chemotherapy in Curative Resected Stage III Gastric Cancer
- Infusional 5-Fluorouracil, Leucovorin and Docetaxel in Advanced Gastric Cancer
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Efficacy and Safety of Bolus 5-Fluorouracil and L-Leucovorin as Salvage Chemotherapy for Oral Fluoropyrimidine-Resistant Unresectable or Recurrent Gastric Cancer: A Single Center Experience
- Phase II Study of Oxaliplatin, 5-fluorouracil, and Leucovorin in Relapsed or Metastatic Colorectal Cancer as Second Line Therapy