Cancer Res Treat.
2008 Jun;40(2):62-70.
Treatment Outcome of Cisplatin-based Concurrent Chemoradiotherapy in the Patients with Locally Advanced Nasopharyngeal Cancer
- Affiliations
-
- 1Head & Neck Cancer Interdisciplinary Team, The Catholic University of Korea College of Medicine, Seoul, Korea. jinkang@catholic.ac.kr
- 2Departments of Diagnostic Radiology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Departments of Nuclear Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Departments of Hospital Pathology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 5Departments of Radiation Oncology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 6Departments of Otorhinolaryngology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 7Departments of Medical Oncology, The Catholic University of Korea College of Medicine, Seoul, Korea.
Abstract
-
PURPOSE: The standard treatment of locally advanced nasopharyngeal cancer is a concurrent chemoradiotherapy (CCRT), and cisplatin has been used as the most popular chemotherapeutic agent. But many different doses and schedules for cisplatin administration such as daily, weekly and 3 week cycles have been proposed. We compared and analyzed the tumor response, the overall survival, the toxicity and the chemotherapy dose intensity in the patients with locally advanced nasopharyngeal cancer who were treated with CCRT.
MATERIALS AND METHODS
We performed a retrospective study on 55 patients with locally advanced nasopharyngeal cancer, and they were treated with CCRT as a front-line treatment from Jan 1996 to Jun 2007 at Kangnam Saint Mary's Hospital.
RESULTS
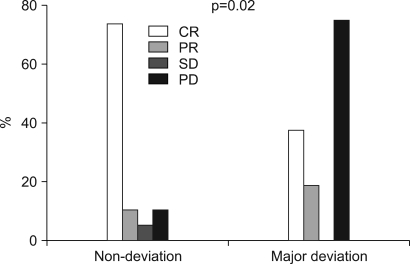
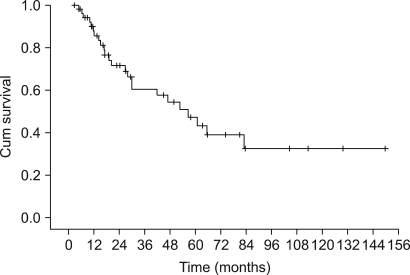
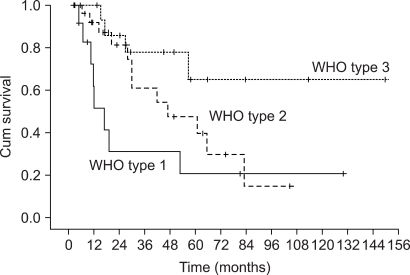
The patients had a median age of 53 years (range: 19~75 years). Of the total 55 patients, a 3-week cycle of 100mg cisplatin was administered in 31 patients and 30 mg weekly cisplatin was administered in 24 patients combined with radiotherapy. Twenty one patients had a complete response and four patients had a partial response for a response rate of 71.4% (95% CI: 59.5~83.3) after CCRT and followed by adjuvant chemo-therapy. The complete response rates for the 30 mg and 100 mg cisplatin groups were 72.7% (95% CI: 54.9~90.5) and 54.2% (95% CI: 36.7~71.7), respectively (p= 0.23). The duration of CCRT in the 100mg cisplatin group was significantly longer than that of the 30mg cisplatin group (11.1+/-2.9 weeks vs. 9.0+/-1.2 weeks, p= 0.003). The major deviation group, which was defined as prolongation of the radiotherapy duration for more than 2 weeks, had a significantly lower objective response rate than did the non-deviation group (56.3% vs 84.2%, respectively, p= 0.002). The major severe toxicities were leucopenia (49.1%), pharyngoesophagitis (49.1%), anorexia (43.6%), nausea (41.8%) and vomiting (40%).
CONCLUSIONS
Weekly 30mg cisplatin-based CCRT is a practical, feasible cisplatin schedule for the patients with locally advanced nasopharyngeal cancer in regard to decreasing the interruption of radiation treatment and decreasing the treatment-related acute toxicities.
Keyword
MeSH Terms
Figure
Reference
-
2. Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol Cancer. 2007; 6:1. PMID: 17199893.3. Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002; 13:1007–1015. PMID: 12176778.
Article4. Qin DX, Hu YH, Yan JH, Xu GZ, Cai WM, Wu XL, et al. Analysis of 1,379 patients with nasopharyngeal carcinoma treated by radiation. Cancer. 1988; 61:1117–1124. PMID: 3342372.
Article5. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998; 16:1310–1317. PMID: 9552031.
Article6. Vokes EE, Weichselbaum RR. Concomitant chemoradiotherapy: Rationale and clinical experience in patients with solid tumors. J Clin Oncol. 1990; 8:911–934. PMID: 2185342.
Article7. Al-Sarraf M. Treatment of locally advanced head and neck cancer: Historical and critical review. Cancer control. 2002; 9:387–399. PMID: 12410178.
Article8. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union Against Cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005; 23:6730–6738. PMID: 16170180.
Article9. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003; 21:631–637. PMID: 12586799.
Article10. Baujat B, Audry H, Bourhis J, Chan AT. Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma (Review). 2007. The Cochrane Collaboration.11. Kwong DL, Sham JS, Chua DT, Choy DT, Au GK, Wu PMl. The effect of interruptions and prolonged treatment time in radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1997; 39:703–710. PMID: 9336153.
Article12. Isobe K, Kawakami H, Uno T, Yasuda S, Aruga T, Ueno N, et al. Concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma: is intergroup study 0099 feasible in Japanese patients? Jpn J Clin Oncol. 2003; 33:497–500. PMID: 14623916.13. Kwong DL, Sham JS, Au GK, Chua DT, Kwong PW, Cheng AC, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: A factorial study. J Clin Oncol. 2004; 22:2643–2653. PMID: 15226332.
Article14. Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Sumitsawan Y, Tharavichitkul E, Sukthomya V, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: Randomized, non-inferiority, open trial. Eur J Cancer. 2007; 43:1399–1406. PMID: 17467265.15. O'Meara WP, Lee N. Advances in nasopharyngeal carcinoma. Curr Opin Oncol. 2005; 17:225–230. PMID: 15818165.16. RTOG acute radiation morbidity scoring criteria. <http://www.rtog.org/members/toxicity/acute.html>.17. Cheng SH, Jian JJ, Tsai SY, Yen KL, Chu NM, Chan NM, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2000; 48:1323–1330. PMID: 11121629.
Article18. Bahl M, Siu LL, Pond GR, Kim J, Tannock IF, Bayley A, et al. Tolerability of the Intergroup 0099 (INT 0099) regimen in locally advanced nasopharyngeal cancer with a focus on patients' nutritional status. Int J Radiat Oncol Biol Phys. 2004; 60:1127–1136. PMID: 15519784.
Article19. Chen YP, Tsang NM, Tseng CK, Lin SY. Causes of interruption of radiotherapy in nasopharyngeal carcinoma patients in Taiwan. Jpn J Clin Oncol. 2000; 30:230–234. PMID: 10857501.
Article20. Reddy SP, Raslan WF, Gooneratne S, Kathuria S. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol. 1995; 16:103–108. PMID: 7540805.
Article21. Marks JE, Phillips JL, Menck HR. The national cancer data base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer. 1998; 83:582–588. PMID: 9690553.
Article22. Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004; 59:11–20. PMID: 15093894.
Article23. Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW, Millward MJ, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patient with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005; 23:3568–3576. PMID: 15809453.24. Hak Choy. Chemoradiation in cancer therapy. 2003. 1st ed. New Jersey: Humana Press;p. 9–50.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Does the Addition of Adjuvant Chemotherapy to Concurrent Chemoradiotherapy Improve the Survival of Patients with Locally Advanced Nasopharyngeal Cancer?
- Comparison of concurrent chemoradiotherapy with cisplatin plus 5-fluorouracil versus cisplatin plus paclitaxel in patients with locally advanced cervical carcinoma
- The Addition of Induction Chemotherapy Failed to Improve Therapeutic Outcome of Concurrent Chemoradiotherapy in Patients with Locally Advanced Non-small Cell Lung Cancer
- Preoperative Concurrent Chemoradiotherapy with Oral Fluoropyrimidine in Locally Advanced Rectal Cancer: How Good Is Good Enough?
- Concurrent Docetaxel/Cisplatin and Thoracic Radiotherapy for Locally Advanced Non-Small Cell Lung Cancer