Cancer Res Treat.
2009 Mar;41(1):19-23.
The Effect of Adjuvant Chemotherapy on Stage IV (T4N1-3M0 and T1-3N3M0) Gastric Cancer
- Affiliations
-
- 1Department of Surgery, Hanyang University College of Medicine, Seoul, Korea. sjkwon@hanyang.ac.kr
Abstract
-
PURPOSE: The optimal chemotherapeutic strategy for gastric cancer patients has not been determined, especially with respect to stage and the curability of gastric cancer. The aim of this study was to evaluate the results of adjuvant chemotherapy on stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer after curative gastrectomy between a chemotherapy (CTX) group and non-chemotherapy (non-CTX) group.
MATERIALS AND METHODS
Among 1,760 patients who underwent gastric surgery by 1 surgeon in a single institution, 162 stage IV gastric cancer patients with curative gastrectomy were analyzed retrospectively, excluding patients with TanyNanyM1. One hundred twenty-five patients who received different chemotherapeutic regimens were compared to 37 patients who did not receive chemotherapy for reasons of old age or according to their expressed desire.
RESULTS
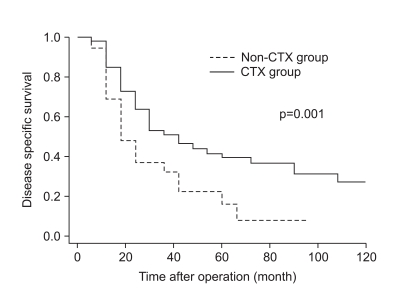
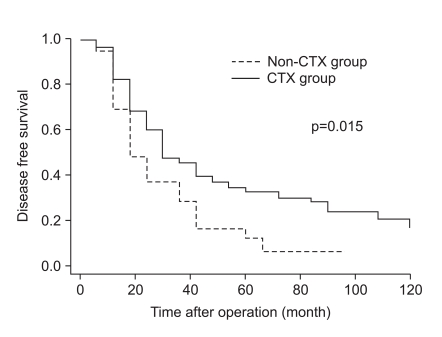
The clinicopathologic factors which showed a clinically significant difference between the two groups were age and histology, which were not associated with patient survival. The CTX group was younger, and had a larger proportion of undifferentiated gastric cancers than the non-CTX group. The mode of treatment failure revealed no significant difference between the CTX and non-CTX groups. The 1, 3, and 5-year disease-free survival and the 1, 3, and 5-year disease-specific survival of the CTX group were 63.9%, 38.4%, and 32.0%, and 85.4%, 52.3%, and 39.6%, respectively, which were more favorable than the non-CTX group (p=0.015 and p=0.001, respectively). Postoperative adjuvant CTX was an independent risk factor for disease-specific survival of stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer patients after curative gastrectomy by multivariate analysis (odds ratio=2.153; 95% confidence interval=1.349-3.435; p=0.001).
CONCLUSIONS
Adjuvant CTX may be associated with survival benefit for younger patients with stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer with undifferentiated histology after curative gastrectomy. A randomized controlled trial to reveal the effect of stage-specific adjuvant chemotherapy should be conducted.
MeSH Terms
Figure
Reference
-
1. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999; 49:33–64. PMID: 10200776.
Article2. Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993; 11:1441–1447. PMID: 8336183.
Article3. Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, et al. Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002; 88:21–27. PMID: 12004845.4. Liu TS, Wang Y, Chen SY, Sun YH. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol. 2008; 34:1208–1216. PMID: 18353606.
Article5. Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol. 2000; 11:837–843. PMID: 10997811.6. Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest. 2008; 26:317–325. PMID: 18317973.
Article7. Greene FL. TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol. 2003; 21:23–29. PMID: 12923913.
Article8. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655. PMID: 7165009.
Article9. Aurello P, D'Angelo F, Rossi S, Bellagamba R, Cicchini C, Nigri G, et al. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am Surg. 2007; 73:359–366. PMID: 17439029.
Article10. Noguchi M, Miyazaki I. Prognostic significance and surgical management of lymph node metastasis in gastric cancer. Br J Surg. 1996; 83:156–161. PMID: 8689153.
Article11. Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst. 2008; 100:388–398. PMID: 18334706.
Article12. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID: 17978289.
Article13. Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, et al. Randomized trial of adjuvant chemotherapy with mitomycin, Fluorouracil, and Cytosine arabinoside followed by oral Fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003; 21:2282–2287. PMID: 12805327.
Article14. Bajetta E, Buzzoni R, Mariani L, Beretta E, Bozzetti F, Bordogna G, et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol. 2002; 13:299–307. PMID: 11886009.
Article15. Neri B, Cini G, Andreoli F, Boffi B, Francesconi D, Mazzanti R, et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br J Cancer. 2001; 84:878–880. PMID: 11286464.
Article16. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20. PMID: 16822992.
Article17. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730. PMID: 11547741.
Article18. Sobrero A. Should adjuvant chemotherapy become standard treatment for patients with stage II colon cancer? For the proposal. Lancet Oncol. 2006; 7:515–516. PMID: 16750502.19. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008; 51:503–507. PMID: 18322753.
Article20. Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007; 94:1468–1476. PMID: 17948223.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Subclassification of Stage IV Gastric Cancer According to the Presence of Distant Metastasis (IVa and IVb)
- Update of Adjuvant Chemotherapy for Resected Gastric Cancer
- Role of Adjuvant Radiotherapy in Gastric Cancer
- Postoperative FP ( 5-Fluorouracil , Cisplatin ) Chemotherapy for Patients with High - Risk Gastric Cancer
- Post-operative Adjuvant Chemotherapy with 5-Fluorouracil, Leucovorin, and Mitomycin C (MLF) for Gastric Cancer