Cancer Res Treat.
2009 Jun;41(2):67-72.
Treatment Outcomes of Sunitinib Treatment in Advanced Renal Cell Carcinoma Patients: A Single Cancer Center Experience in Korea
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. rha7655@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea.
Abstract
- PURPOSE
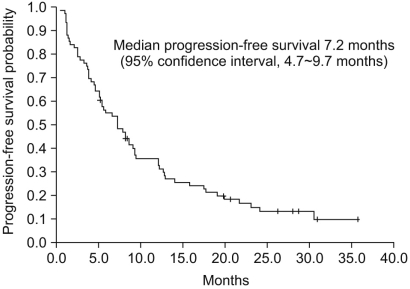
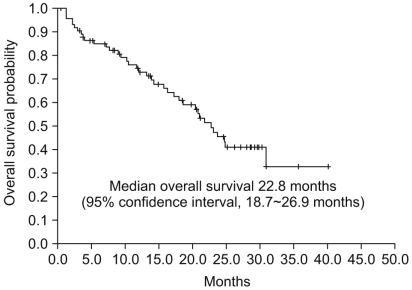
The retrospective study was performed to assess the efficacy and toxicity profiles of sunitinib in Korean patients with metastatic renal cell carcinoma (RCC). MATERIALS AND METHODS: Between January 2005 and December 2008, 76 Korean patients with recurrent/metastatic RCC who received sunitinib were retrospectively reviewed. The primary end point was progression-free survival and the secondary end points were overall survival and response rate. We also assessed the toxicities associated with sunitinib treatment. RESULTS: Of the 76 patients, 69 (90.1%) were diagnosed with clear cell RCC. The median progression-free survival and overall survival were 7.2 and 22.8 months, respectively in overall patients. Sixty-two patients (81.6%) received 50 mg 4 week and 2 week off schedule, and 14 patients (18.4%) received 37.5 mg daily on a daily continuous schedule. The objective response rate and disease control rate were 27.6% and 84.2%, respectively. A dose reduction or reduction in dose due to adverse events occurred in 76% of the patients, whereas 11% of the patients had discontinued treatment. Other common laboratory abnormalities were increased serum creatinine (75.6%), elevated alanine aminotransferase (71.0%), neutropenia (61.8%), anemia (69.7%), and increased aspartate aminotrasferase (53.3%). Grade 3/4 toxicities occurred as follows: thrombocytopenia (38.2%), fatigue (10.5%), stomatitis (10.5%), and hand-foot syndrome (9.2%). CONCLUSION: Our results indicate that sunitinib treatment is effective and tolerable for ecurrent/metastatic RCC patients in Korea. Further studies with prognostic or biochemical factors are needed to clarify the different toxicity profiles of this study.
Keyword
MeSH Terms
-
Alanine Transaminase
Anemia
Appointments and Schedules
Aspartic Acid
Carcinoma, Renal Cell
Creatinine
Disease-Free Survival
Fatigue
Hand-Foot Syndrome
Humans
Indoles
Korea
Neutropenia
Pyrroles
Retrospective Studies
Stomatitis
Thrombocytopenia
Alanine Transaminase
Aspartic Acid
Carcinoma, Renal Cell
Creatinine
Indoles
Pyrroles
Figure
Reference
-
1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–2150. PMID: 16682732.
Article2. Jemal A, Murray T, Ward E, Samuels A, Tiwari R, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005; 55:10–30. PMID: 15661684.
Article3. Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003; 30:843–852. PMID: 14680319.
Article4. Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007; 25:884–896. PMID: 17327610.
Article5. Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 Inhibits KIT and platelet-derived growth factor receptor β, in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003; 2:471–478. PMID: 12748309.6. Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994; 7:85–90. PMID: 7915601.
Article7. Iliopoulos O, Levy AP, Jiang C, Kaelin WG Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996; 93:10595–10599. PMID: 8855223.
Article8. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon α in metastatic renal-cell carcinoma. N Engl J Med. 2007; 356:115–124. PMID: 17215529.9. Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004; 22:454–463. PMID: 14752067.
Article10. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.
Article11. Choi MK, Hong JY, Jang JH, Lim HY. TTP-HUS associated with sunitinib. Cancer Res Treat. 2008; 40:211–213. PMID: 19688133.
Article12. Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006; 295:2516–2524. PMID: 16757724.
Article13. Uemura H, Shinohara N, Naito S, Akaza H. A phase II study of the efficacy and safety of sunitinib in treatment-naive and pretreated Japanese patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2008; 26:15S. (Abstr 16108).14. Law CC, Fu YT, Chau KK, Choy TS, So PF, Wong KH. Toxicity profile and efficacy of oral capecitabine as adjuvant chemotherapy for Chinese patients with stage III colon cancer. Dis Colon Rectum. 2007; 50:2180–2187. PMID: 17963003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sunitinib-induced hypothyroidism
- Intolerance to Sunitinib Treatment in Hemodialysis Patients With Metastatic Renal Cell Carcinoma
- Sunitinib-induced hypothyroidism
- Hypoglycemic Coma in a Patient with Metastatic Renal Cell Carcinoma Treated with Sunitinib
- Novel Sunitinib Strategy in Metastatic Renal Cell Carcinoma on Hemodialysis: Intermittent Dose of Sunitinib after Hemodialysis