Ann Rehabil Med.
2014 Jun;38(3):327-334. 10.5535/arm.2014.38.3.327.
Characteristics of Neuropathic Pain in Patients With Spinal Cord Injury
- Affiliations
-
- 1Department of Rehabilitation Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea. jseok337@cha.ac.kr
- KMID: 2165749
- DOI: http://doi.org/10.5535/arm.2014.38.3.327
Abstract
OBJECTIVE
To characterize neuropathic pain in patients with spinal cord injury (SCI) according to classification used in the study by Baron et al. (Baron classification), a classification of neuropathic pain based on the mechanism. To also compare the patterns of neuropathic pain in SCI patients with those in patients with other etiologies and to determine the differences in patterns of neuropathic pain between the etiologies.
METHODS
This was a descriptive cross-sectional study. We used the Baron classification to investigate the characteristics of neuropathic pain in SCI. Sixty-one SCI patients with neuropathic pain (The Leeds assessment of neuropathic symptoms and signs score > or =12) were enrolled in this study between November 2012 and August 2013, after excluding patients <20 of age, patients with visual analog scale (VAS) score <3, pregnant patients, and patients with systemic disease or pain other than neuropathic pain.
RESULTS
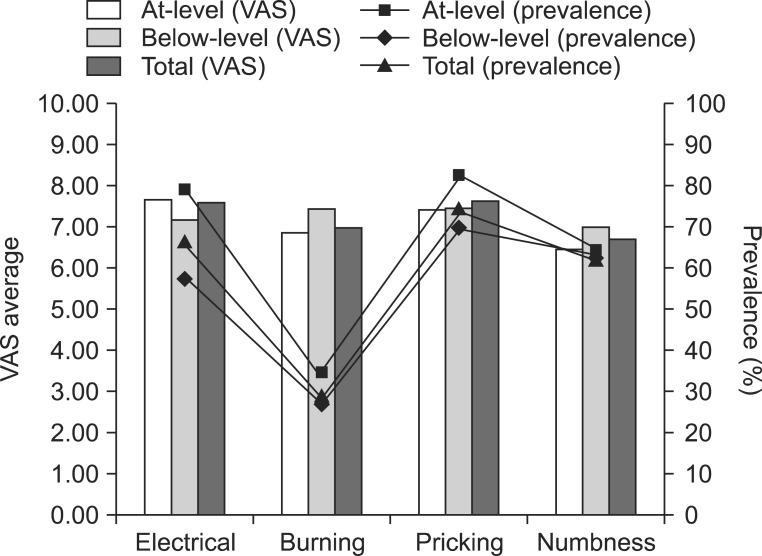
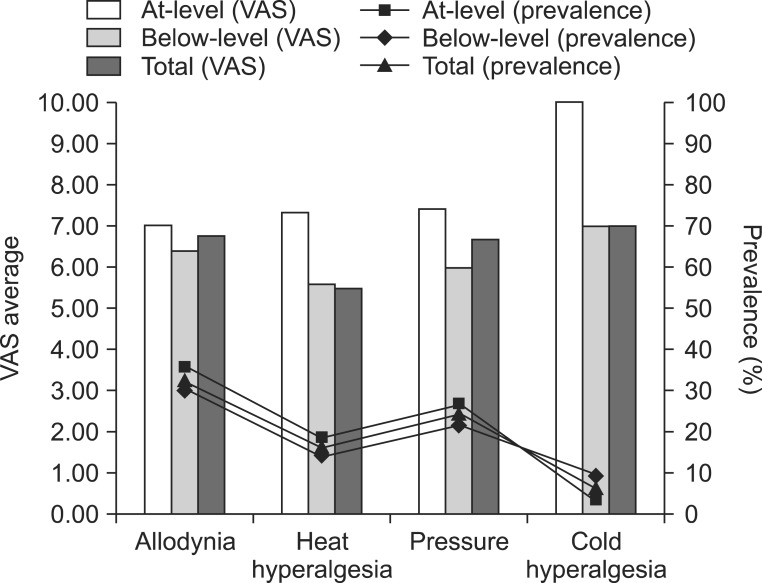
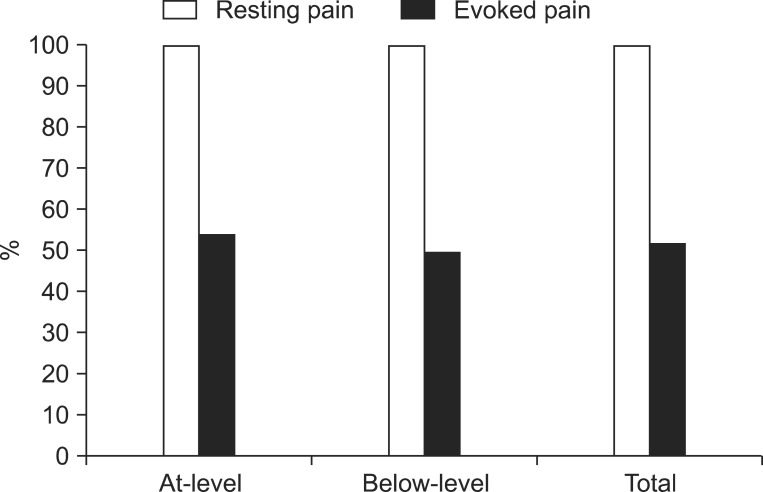
The most common pain characteristic was pricking pain followed by electrical pain and numbness. The mean VAS score of at-level neuropathic pain was 7.51 and that of below-level neuropathic pain was 6.83. All of the patients suffered from rest pain, but 18 (54.6%) patients with at-level neuropathic pain and 20 (50.0%) patients with below-level neuropathic pain suffered from evoked pain. There was no significant difference in between at-level and below-level neuropathic pains.
CONCLUSION
The result was quite different from the characteristics of post-herpetic neuralgia, but it was similar to the characteristics of diabetic neuropathy as shown in the study by Baron et al., which means that sensory nerve deafferentation may be the most common pathophysiologic mechanism of neuropathic pain after SCI. Since in our study, we included short and discrete symptoms and signs based on diverse mechanisms, our results could be helpful for determining further evaluation and treatment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Prevalence and Characteristics of Neuropathic Pain in Patients With Spinal Cord Injury Referred to a Rehabilitation Center
Hae Young Kim, Hye Jin Lee, Tae-lim Kim, EunYoung Kim, Daehoon Ham, Jaejoon Lee, Tayeun Kim, Ji Won Shin, Minkyoung Son, Jun Hun Sung, Zee-A Han
Ann Rehabil Med. 2020;44(6):438-449. doi: 10.5535/arm.20081.
Reference
-
1. Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007; 21:89–96. PMID: 17213246.
Article2. Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine (Phila Pa 1976). 2001; 26(24 Suppl):S146–S160. PMID: 11805622.3. Westgren N, Levi R. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil. 1998; 79:1433–1439. PMID: 9821906.
Article4. Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007; 23:383–391. PMID: 17515736.
Article5. Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Follinger S, Wienke C, et al. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997; 35:446–455. PMID: 9232750.
Article6. Celik EC, Erhan B, Lakse E. The clinical characteristics of neuropathic pain in patients with spinal cord injury. Spinal Cord. 2012; 50:585–589. PMID: 22430511.
Article7. Fenollosa P, Pallares J, Cervera J, Pelegrin F, Inigo V, Giner M, et al. Chronic pain in the spinal cord injured: statistical approach and pharmacological treatment. Paraplegia. 1993; 31:722–729. PMID: 7507585.
Article8. Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008; 70:1630–1635. PMID: 18003941.
Article9. Ro LS, Chang KH. Neuropathic pain: mechanisms and treatments. Chang Gung Med J. 2005; 28:597–605. PMID: 16323550.10. Widerstrom-Noga EG, Turk DC. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord. 2003; 41:600–609. PMID: 14569261.
Article11. Baron R, Tolle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. Pain. 2009; 146:34–40. PMID: 19592166.
Article12. Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001; 92:147–157. PMID: 11323136.
Article13. Bryce TN, Ragnarsson KT. Pain after spinal cord injury. Phys Med Rehabil Clin N Am. 2000; 11:157–168. PMID: 10680163.
Article14. Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord. 1997; 35:69–75. PMID: 9044512.
Article15. Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research: report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J Spinal Cord Med. 2007; 30:421–440. PMID: 18092558.16. Bryce TN, Dijkers MP, Ragnarsson KT, Stein AB, Chen B. Reliability of the Bryce/Ragnarsson spinal cord injury pain taxonomy. J Spinal Cord Med. 2006; 29:118–132. PMID: 16739555.
Article17. Finnerup NB, Jensen TS. Mechanisms of disease: mechanism-based classification of neuropathic paina critical analysis. Nat Clin Pract Neurol. 2006; 2:107–115. PMID: 16932532.
Article18. Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, et al. Towards a mechanism-based classification of pain? Pain. 1998; 77:227–229. PMID: 9808347.
Article19. Freynhagen R, Baron R, Gockel U, Tolle TR. painDE TECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006; 22:1911–1920. PMID: 17022849.20. Baron R. Mechanisms of disease: neuropathic pain: a clinical perspective. Nat Clin Pract Neurol. 2006; 2:95–106. PMID: 16932531.21. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010; 9:807–819. PMID: 20650402.
Article22. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999; 353:1959–1964. PMID: 10371588.
Article23. Harden RN. Chronic neuropathic pain: mechanisms, diagnosis, and treatment. Neurologist. 2005; 11:111–122. PMID: 15733333.24. Calmels P, Mick G, Perrouin-Verbe B, Ventura M. French Society for Physical Medicine and Rehabilitation. Neuropathic pain in spinal cord injury: identification, classification, evaluation. Ann Phys Rehabil Med. 2009; 52:83–102. PMID: 19909700.
Article25. Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003; 25:81–104. PMID: 12637113.
Article26. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998; 280:1831–1836. PMID: 9846777.
Article27. Rice AS, Maton S. Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001; 94:215–224. PMID: 11690735.
Article28. Caraceni A, Zecca E, Martini C, De Conno F. Gabapentin as an adjuvant to opioid analgesia for neuropathic cancer pain. J Pain Symptom Manage. 1999; 17:441–445. PMID: 10388250.
Article29. Dworkin RH, Corbin AE, Young JP Jr, Sharma U, LaMoreaux L, Bockbrader H, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003; 60:1274–1283. PMID: 12707429.
Article30. Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005; 115:254–263. PMID: 15911152.
Article31. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004; 110:628–638. PMID: 15288403.
Article32. Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006; 67:1792–1800. PMID: 17130411.
Article33. Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996; 65:39–44. PMID: 8826488.
Article34. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004; 110:697–706. PMID: 15288411.
Article35. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001; 57:1583–1588. PMID: 11706096.
Article36. McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006; 10:127–135. PMID: 16310716.
Article37. O'Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009; 27:95–112. PMID: 19254044.38. Tolle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006; 20:26–33. PMID: 16389164.39. O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009; 122(10 Suppl):S22–S32. PMID: 19801049.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinal Gap Junction Channels in Neuropathic Pain
- Intractable Abdominal Pain in a Patient With Spinal Cord Injury: A Case Report
- Posterior Cord Syndrome After Spinal Cord Stimulation Electrode Lead Insertion: A Case Report
- Management of Spinal Cord Injury Pain with Small Divided Doses of Intravenous Ketamine
- Spinal cord stimulation for neuropathic pain following idiopathic transverse myelitis: A case report