Ann Rehabil Med.
2014 Aug;38(4):433-442. 10.5535/arm.2014.38.4.433.
Effects of Bladder Function by Early Tamsulosin Treatment in a Spinal Cord Injury Rat Model
- Affiliations
-
- 1Department of Rehabilitation Medicine and Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea. mcjoo68@wku.ac.kr
- 2Department of Physiology, Wonkwang University School of Medicine, Iksan, Korea.
- 3Department of Urology, Wonkwang University School of Medicine, Iksan, Korea.
- KMID: 2165725
- DOI: http://doi.org/10.5535/arm.2014.38.4.433
Abstract
OBJECTIVE
To investigate the effects of early tamsulosin treatment on changes in bladder characteristics after a spinal cord injury.
METHODS
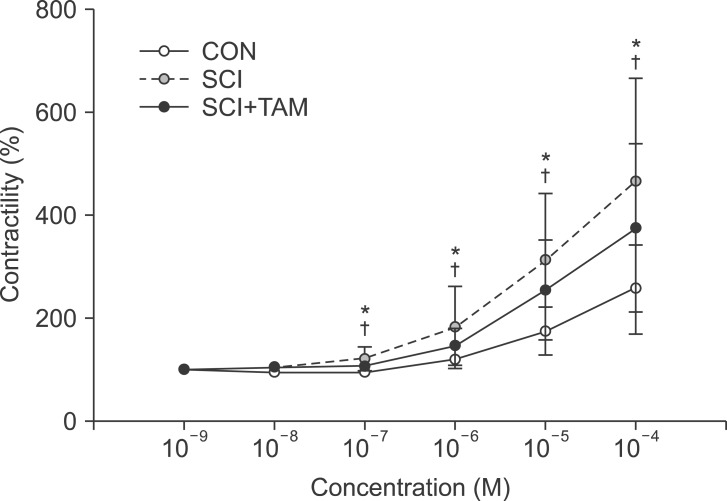
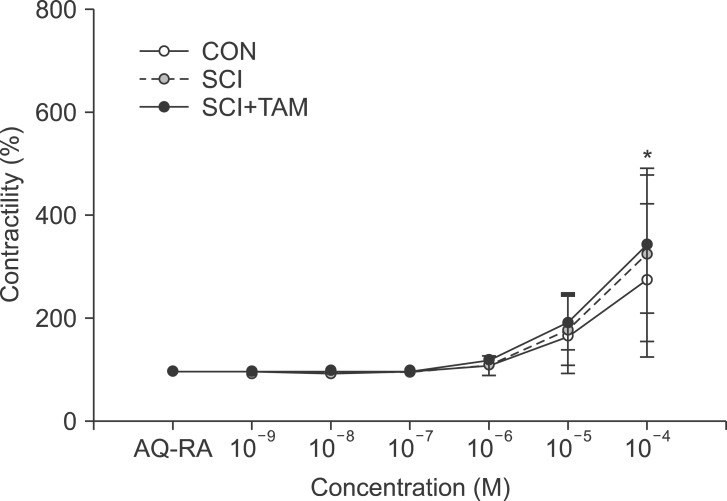
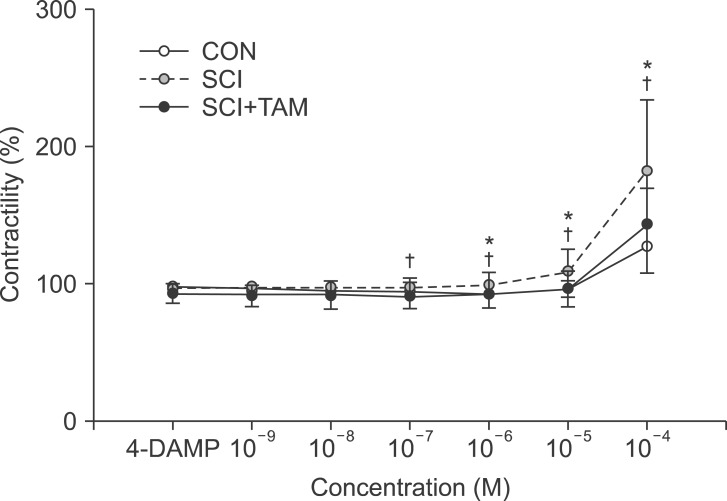
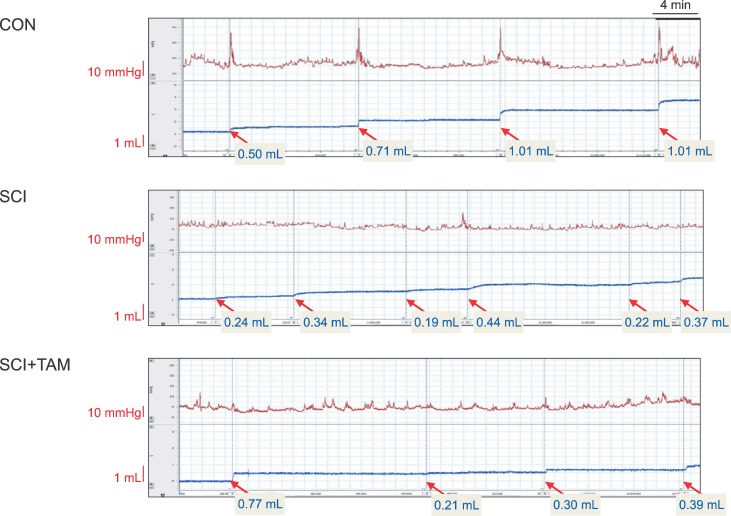
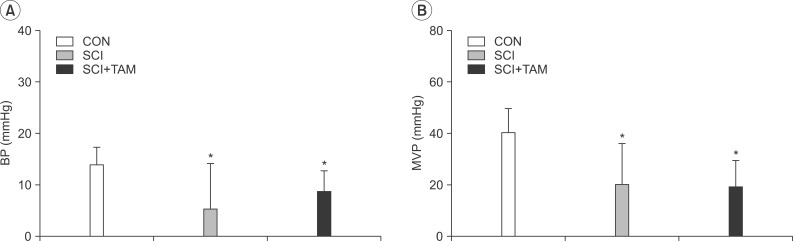
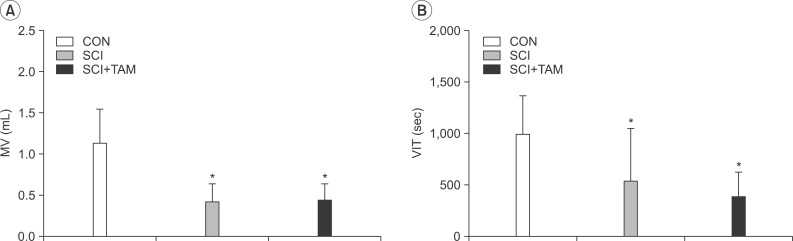
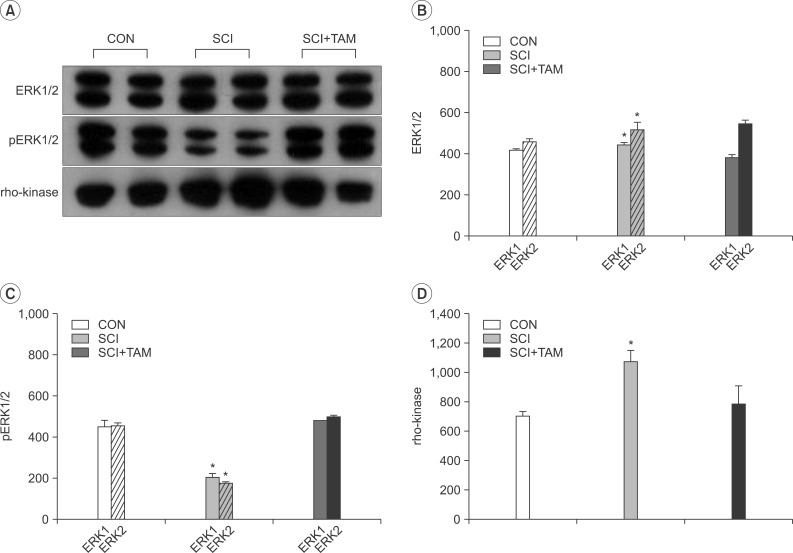
We divided 45 rats into three groups: the control (CON) group, the spinal cord injury (SCI) group, and the SCI+tamsulosin treatment (SCI+TAM) group. Spinal cord transection was performed in the SCI and SCI+TAM groups. Tamsulosin was injected for 7 days in the SCI+TAM group. Intravesical and intra-abdominal catheters were implanted before cord injury. Basal pressure (BP), maximal vesical pressure (MVP), micturition volume (MV), and voiding interval time (VIT) were measured at 7 days after SCI. The bladder was then removed and used for an in vitro organ bath study and Western blot analysis. The percentage changes in contractility from baseline after acetylcholine alone, pretreatment with a muscarinic 2 (M2) receptor blocker (AQ-RA741), and pretreatment with a M3 receptor blocker (4-DAMP) were compared among the groups. Western blot analyses were performed to determine expression levels of pERK1/2 and rho-kinase.
RESULTS
In cystometry, MVP, BP, MV, and VIT showed changes in the SCI and SCI+TAM groups versus the CON group (p<0.05). In the organ bath study, acetylcholine-induced contractility in the three groups differed significantly (p<0.05). Additionally, acetylcholine-induced contractility with 4-DAMP pretreatment was reduced significantly in the SCI+TAM group versus the SCI group. In Western blotting, pERK1/2 expression was stronger (p<0.05) and rho-kinase expression was weaker in the SCI+TAM group than the SCI group (p<0.05).
CONCLUSION
These results suggest that the bladder contraction due to acetylcholine after SCI can be decreased by tamsulosin in the acute stage and this involves changes in pERK1/2 and rho-kinase.
MeSH Terms
Figure
Reference
-
1. Kakizaki H, Koyanagi T. Current view and status of the treatment of lower urinary tract symptoms and neurogenic lower urinary tract dysfunction. BJU Int. 2000; 85(Suppl 2):25–30. PMID: 10781182.
Article2. de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997; 50(6A Suppl):36–56. PMID: 9426749.
Article3. Braverman AS, Ruggieri MR Sr. Hypertrophy changes the muscarinic receptor subtype mediating bladder contraction from M3 toward M2. Am J Physiol Regul Integr Comp Physiol. 2003; 285:R701–R708. PMID: 12763741.4. Nickel JC. The use of alpha1-adrenoceptor antagonists in lower urinary tract symptoms: beyond benign prostatic hyperplasia. Urology. 2003; 62(3 Suppl 1):34–41. PMID: 12957198.5. Price D. Potential mechanisms of action of superselective alpha(1)-adrenoceptor antagonists. Eur Urol. 2001; 40(Suppl 4):5–11. PMID: 11786674.6. Lee T, Andersson KE, Streng T, Hedlund P. Simultaneous registration of intraabdominal and intravesical pressures during cystometry in conscious rats: effects of bladder outlet obstruction and intravesical PGE2. Neurourol Urodyn. 2008; 27:88–95. PMID: 17565725.7. Anderson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993; 45:253–308. PMID: 8248281.8. Swierzewski SJ 3rd, Gormley EA, Belville WD, Sweetser PM, Wan J, McGuire EJ. The effect of terazosin on bladder function in the spinal cord injured patient. J Urol. 1994; 151:951–954. PMID: 7907374.
Article9. Perlberg S, Caine M. Adrenergic response of bladder muscle in prostatic obstruction: its relation to detrusor instability. Urology. 1982; 20:524–527. PMID: 7147531.10. Hampel C, Dolber PC, Smith MP, Savic SL, Th roff JW, Thor KB, et al. Modulation of bladder alpha1-adrenergic receptor subtype expression by bladder outlet obstruction. J Urol. 2002; 167:1513–1521. PMID: 11832780.11. Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010; 67:405–417. PMID: 19862476.12. Robinson D, Cardozo L, Terpstra G, Bolodeoku J. Tamsulosin Study Group. A randomized double-blind placebo-controlled multicentre study to explore the efficacy and safety of tamsulosin and tolterodine in women with overactive bladder syndrome. BJU Int. 2007; 100:840–845. PMID: 17822465.
Article13. Chapple CR. Alpha-adrenergic blocking drugs in bladder outflow obstruction: what potential has alpha 1-adrenoceptor selectivity? Br J Urol. 1995; 76(Suppl 1):47–55. PMID: 7544214.14. Abrams P, Amarenco G, Bakke A, Buczynski A, Castro-Diaz D, Harrison S, et al. Tamsulosin: efficacy and safety in patients with neurogenic lower urinary tract dysfunction due to suprasacral spinal cord injury. J Urol. 2003; 170(4 Pt 1):1242–1251. PMID: 14501734.
Article15. de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990; 30(Suppl):S71–S77. PMID: 2212495.
Article16. Caulfield MP. Muscarinic receptors: characterization, coupling and function. Pharmacol Ther. 1993; 58:319–379. PMID: 7504306.17. Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998; 50:279–290. PMID: 9647869.18. Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999; 64:419–428. PMID: 10069505.
Article19. Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002; 22:133–145. PMID: 12452898.
Article20. Tong YC, Chin WT, Cheng JT. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocin-induced diabetic rats. Neurosci Lett. 1999; 277:173–176. PMID: 10626841.
Article21. Sibley GN. The physiological response of the detrusor muscle to experimental bladder outflow obstruction in the pig. Br J Urol. 1987; 60:332–336. PMID: 3690205.
Article22. German K, Bedwani J, Davies J, Brading AF, Stephenson TP. Physiological and morphometric studies into the pathophysiology of detrusor hyperreflexia in neuropathic patients. J Urol. 1995; 153:1678–1683. PMID: 7715009.
Article23. Cook AK, Carty M, Singer CA, Yamboliev IA, Gerthoffer WT. Coupling of M(2) muscarinic receptors to ERK MAP kinases and caldesmon phosphorylation in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2000; 278:G429–G437. PMID: 10712263.24. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996; 271:20246–20249. PMID: 8702756.
Article25. Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997; 272:12257–12260. PMID: 9139666.
Article26. Sung JK, Miao L, Calvert JW, Huang L, Louis Harkey H, Zhang JH. A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Res. 2003; 959:29–38. PMID: 12480155.
Article27. Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, et al. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem. 2009; 284:11454–11466. PMID: 19261619.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinal Cord Injury Rehabilitation (II): Management of Neurogenic Bladder
- Effect of ABT-594 a Selective Nicotinic Agonist, on Voiding Function in Spinal Cord Injury Rat
- Changes of BDNF (Brain Derived Neurotrophic Factor) Expression Associated with Urodynamic Changes in Rat Spinal Cord Injury Animal Model
- The Effects of Intravesical Oxybutynin Chloride in Spinal Cord Injury Patients Who Had Clinical Problems on Oral Medication
- Changes in the Expression of Smooth Muscle Myosin Heavy Chain mRNA following Partial Bladder Obstruction or Spinal Cord Injury in Rat: A Preliminary Study