Brain Neurorehabil.
2015 Sep;8(2):96-103. 10.12786/bn.2015.8.2.96.
Comparison of Functions, Activity of Daily Living, and Quality of Life according to Hand Dominance in Stroke
- Affiliations
-
- 1Department of Rehabilitation Medicine, Pusan National University School of Medicine, Korea. ijsh6679@gmail.com, rmshin@pusan.ac.kr
- 2Research Institute of Convergence for Biomedical Science and Technology, Pusan National University Yangsan Hospital, Korea.
- 3Division of Biostatistics, Research Institute of Convergence for Biomedical Science and Technology, Pusan National University Yangsan Hospital, Korea.
- 4Department of Rehabilitation Medicine, Pusan National University Yangsan Hospital, Korea.
- 5Department of Physical and Rehabilitation Medicine, Center for Prevention and Rehabilitation, Heart Vascular and Stroke Institute, Samsung Medical Center, Korea.
- 6Department of Physical and Rehabilitation Medicine, Sungkyunkwan University School of Medicine, Korea.
- 7Department of Health Sciences and Technology, Samsung Advanced Institute for health Science and Technology, Sungkyunkwan University, Korea.
- 8Department of Rehabilitation Medicine, Pusan National University Hospital, Korea.
- KMID: 2165197
- DOI: http://doi.org/10.12786/bn.2015.8.2.96
Abstract
OBJECTIVE
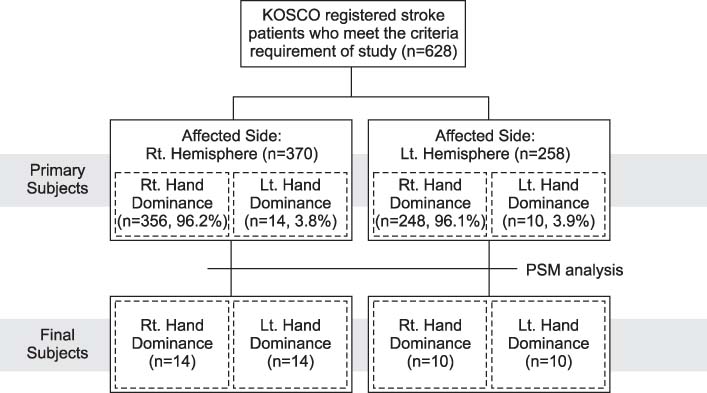
We investigated the differences of functions, activity of daily living (ADL), and quality of life (QoL) according to hand dominance in hemiplegic stroke patients. METHOD: The participants were diagnosed as stroke at P hospital. 370 participants (356 right dominant hand, 14 left dominant hand) were right hemisphere stroke, and 258 participants (248 right dominant hand, 10 left dominant hand) were left hemisphere stroke. To compensate the differences of imbalance in the number of participants' hand dominance, we performed the propensity score matching analysis. We analyzed the variation of stroke severity, disability, motor, mobility, cognition and language functions from 7 days until 3 months after onset using analysis of covariance (ANCOVA). Then, we performed independent t-test to compare hand dexterity, ADL, mood, subjective health condition and QoL of participants according to the hand dominance and the affected hemisphere.
RESULTS
All groups showed increased functions during 3 months without statistically significant differences according to hand dominance in both right and left hemisphere stroke patients. In addition, ADL, mood, subjective health condition and QoL were also not significantly different depending on hand dominance in the right and left hemisphere.
CONCLUSION
The difference of hand dominance did not influence stroke severity, disability, motor, mobility, cognition or language functions. It also didn't correlate with ADLs, mood or QoL.
Keyword
Figure
Reference
-
1. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011; 377:1693–1702.
Article2. Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther. 2013; 26:104–115.
Article3. Schell BA, Gillen G, Scaffa M, Cohn ES. Willard and Spackman's occupational therapy. Lippincott Williams & Wilkins;2013.4. Lavrysen A, Heremans E, Peeters R, Wenderoth N, Feys P, Swinnen SP, Helsen WF. Hemispheric asymmetries in goal-directed hand movements are independent of hand preference. Neuroimage. 2012; 62:1815–1824.
Article5. Meugnot A, Toussaint L. Functional plasticity of sensorimotor representations following short-term immobilization of the dominant versus non-dominant hands. Acta Psychol (Amst). 2015; 155:51–55.
Article6. Corey DM, Hurley MM, Foundas AL. Right and left handedness defined: a multivariate approach using hand preference and hand performance measures. Neuropsychiatry Neuropsychol Behav Neurol. 2001; 14:144–152.7. Provins KA. The Specificity of Motor Skill and Manual Asymmetry: A Review of the Evidence and Its Implications. J Mot Behav. 1997; 29:183–192.
Article8. Bryden M. Laterality functional asymmetry in the intact brain. Elsevier;2012.9. Vega-Gonzalez A, Bain BJ, Dall PM, Granat MH. Continuous monitoring of upper-limb activity in a free-living environment: a validation study. Med Biol Eng Comput. 2007; 45:947–956.
Article10. Rinehart JK, Singleton RD, Adair JC, Sadek JR, Haaland KY. Arm use after left or right hemiparesis is influenced by hand preference. Stroke. 2009; 40:545–550.
Article11. Collins RL. When left-handed mice live in right-handed worlds. Science. 1975; 187:181–184.
Article12. Michel GF. Right-handedness: a consequence of infant supine head-orientation preference? Science. 1981; 212:685–687.
Article13. Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, Oh GJ, Lee YS, Joo MC, Han EY, Kim YH. Korean Stroke Cohort for functioning and rehabilitation (KOSCO): study rationale and protocol of a multi-centre prospective cohort study. BMC neurology. 2015; 15:42.
Article14. Caspers K, Arndt S, Yucuis R, McKirgan L, Spinks R. Effects of alcohol-and cigarette-use disorders on global and specific measures of cognition in middle-age adults. J Stud Alcohol Drugs. 2010; 71:192–200.
Article15. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9:97–113.
Article16. Edlin JM, Leppanen ML, Fain RJ, Hacklander RP, Hanaver-Torrez SD, Lyle KB. On the use (and misuse?) of the Edinburgh Handedness Inventory. Brain Cogn. 2015; 94:44–51.
Article17. Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989; 20:864–870.
Article18. Oh MS, Yu KH, Lee JH, Jung S, Ko IS, Shin JH, Cho SJ, Choi HC, Kim HH, Lee BC. Validity and reliability of a korean version of the national institutes of health stroke scale. J Clin Neurol. 2012; 8:177–183.
Article19. Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975; 7:13–31.20. Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007; 88:1314–1319.
Article21. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–198.22. Enderby PM, Wood VA, Wade DT, Hewer RL. The Frenchay Aphasia Screening Test: a short, simple test for aphasia appropriate for non-specialists. Int Rehabil Med. 1987; 8:166–170.
Article23. Earhart GM, Cavanaugh JT, Ellis T, Ford MP, Foreman KB, Dibble L. The 9-hole PEG test of upper extremity function: average values, test-retest reliability, and factors contributing to performance in people with Parkinson disease. J Neurol Phys Ther. 2011; 35:157–163.24. Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, Kim SH, Kim TH, Han TR. Development of the Korean Version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007; 31:283–297.25. Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993; 74:531–536.
Article26. Greiner W, Claes C, Busschbach JJ, von der Schulenburg JM. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ. 2005; 6:124–130.
Article27. Yesavage J, Brink T, Rose T. Geriatric depression scale (GDS). Handbook of psychiatric measures. Washington DC: American Psychiatric Association;2000. p. 544–546.28. Bethoux F, Calmels P, Gautheron V. CHANGES IN THE QUALITY OF LIFE OF HEMIPLEGIC STROKE PATIENTS WITH TIME: A Preliminary Report1. Am J Phys Med Rehabil. 1999; 78:19–23.29. Cash M, Whittingham K. What facets of mindfulness contribute to psychological well-being and depressive, anxious, and stress-related symptomatology? Mindfulness. 2010; 1:177–182.
Article30. Kim SI, Kim WS, Cho KJ. The type of handedness and correlation analysis of handedness assessment items on university students in Korea. Korean J Phys Anthropol. 2008; 21:245–253.
Article31. Steenhuis RE, Bryden M. Different dimensions of hand preference that relate to skilled and unskilled activities. Cortex. 1989; 25:289–304.
Article32. Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975; 11:230–238.
Article33. Hatta T, Nakatsuka Z. Note on hand preference of Japanese people. Percept Mot Skills. 1976; 42:530–530.
Article34. McManus I. The history and geography of human handedness. Language lateralisation and psychosis. 2009. p. 37–57.35. Nam HU, Huh JS, Yoo JN, Hwang JM, Lee BJ, Min YS, Kim CH, Jung TD. Effect of dominant hand paralysis on quality of life in patients with subacute stroke. Ann Rehabil Med. 2014; 38:450–457.
Article36. Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. 2006; 20:380–338.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activity of Daily Living, Interpersonal Relationship, Depression and Health-related Quality of Life in Patients with Hand Microsurgery
- Factors Associated Quality of Life of Elderly in Non-paid or Paid Assisted Living Facilities
- Factors related to the Health related Quality of Life among Institutionalized Elders
- Quality of Life, Perceived Health Status, Pain, and Difficulty of Activity of Daily Living of Degenerative Arthritis Patient in Island Residents
- A Correlational Study on Activities of Daily Living, Self-efficacy, Stroke Specific Quality of Life and Need for Self-help Management Programs for Patients with Hemiplegia at Home