Int J Thyroidol.
2016 May;9(1):19-28. 10.11106/ijt.2016.9.1.19.
High Serum Levels of Thyroid-Stimulating Hormone and Sustained Weight Gain in Patients with Thyroid Cancer Undergoing Radioiodine Therapy
- Affiliations
-
- 1Department of Nuclear Medicine, Korea University College of Medicine, Seoul, Korea.
- 2Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, Korea. jkchung@snu.ac.kr
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 4Department of Molecular Medicine and Biopharmaceutical Sciences, WCU Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea.
- 5University of California, Irvine, California, USA.
- KMID: 2164912
- DOI: http://doi.org/10.11106/ijt.2016.9.1.19
Abstract
- BACKGROUND AND OBJECTIVES
The extent of weight gain and its association with clinical factors in patients undergoing radioiodine therapy for differentiated thyroid cancer remain unclear. We analyzed clinical factors related to sustained weight gain after serum thyroid-stimulating hormone (TSH) stimulation for radioiodine (I-131) therapy.
MATERIALS AND METHODS
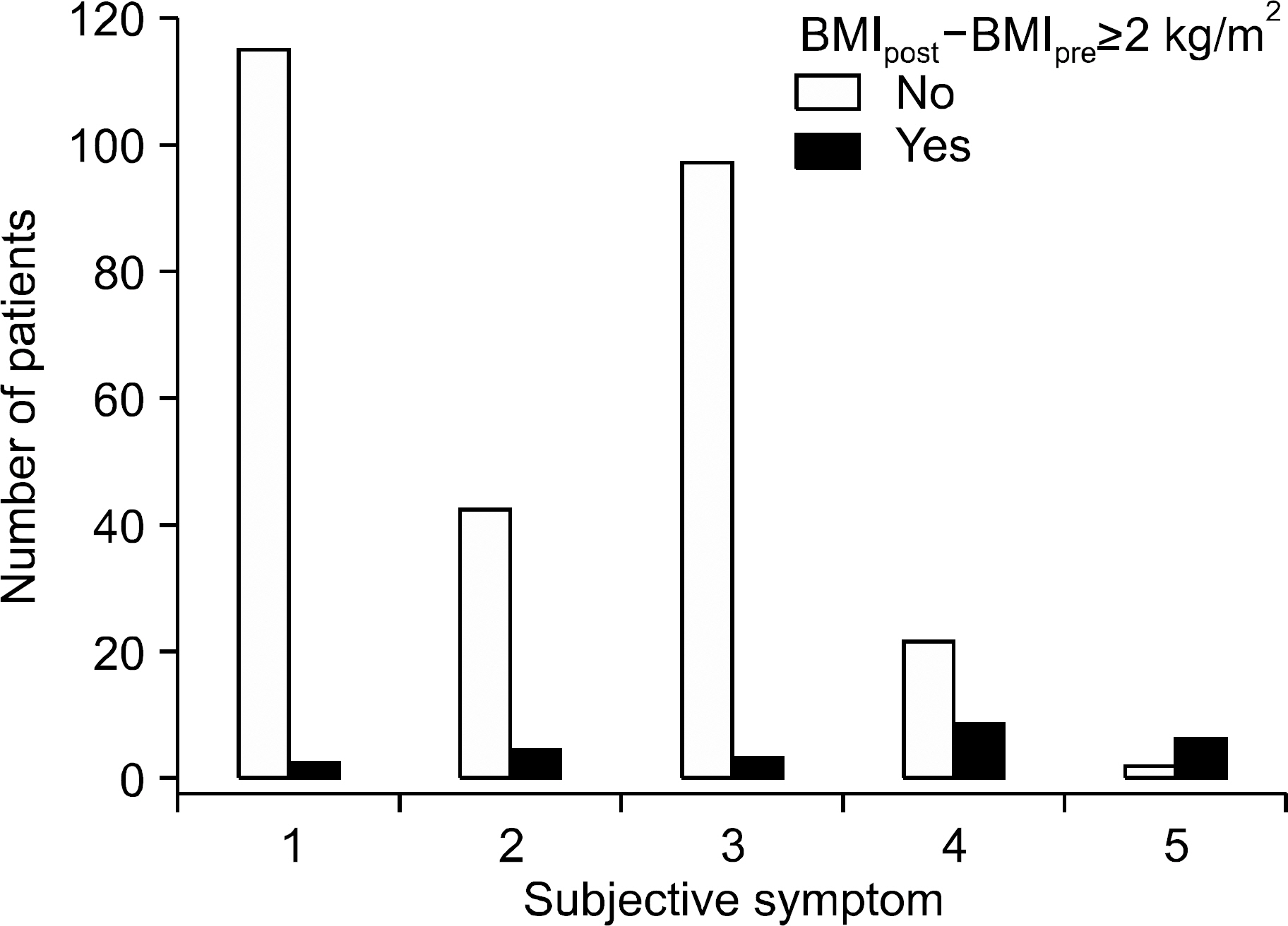
The study population included 301 adult patients who underwent total thyroidectomy followed by radioiodine therapy and visited the thyroid clinic regularly. Group 1 received a single radioiodine therapy treatment, while group 2 received multiple radioiodine treatment. Data on transient weight gain, defined as weight gain that resolved (±5%) within 1 year after radioiodine therapy, were collected from medical records. Sustained weight gain was defined as body mass index after treatment (BMI(post)) - BMI before treatment (BMI(pre)) ≥2 kg/m2 more than 1 year following radioiodine therapy. Subjective symptoms were scored by questionnaire. Logistic regression analysis was performed using various clinical and laboratory factors to identify risk factors associated with sustained weight gain.
RESULTS
Two hundred and fifty-nine (86%) patients showed transient weight gain and 23 (8%) patients showed sustained weight gain. TSH at therapy and T4-on TSH differed significantly in all patients and in the patients in group 1 with sustained weight gain. The proportion of patients with basal BMI≥25 kg/m2 in group 1 with sustained weight gain also differed significantly. Univariate analysis revealed that high serum levels of TSH at therapy (≥100 µIU/mL) and hypercholesterolemia were associated with sustained weight gain in group 1. Multivariate analysis showed that TSH at therapy levels ≥100 µIU/mL was associated with sustained weight gain in group 1. Of 283 patients remaining after excluding those with insufficient TSH suppression during follow-up, T4-on TSH levels were lower in the sustained weight gain group compared to those without sustained weight gain. TSH at therapy levels ≥100 µIU/mL were significantly associated with sustained weight gain in multivariate analysis.
CONCLUSION
Most patients (86%) had transient weight gain after TSH at therapy, while 8% of patients showed sustained weight gain. Univariate and multivariate analysis revealed relatively high TSH levels (≥100 µIU/mL) to be a risk factor for patients that received a single dose of radioiodine therapy. Insufficient T4 dose was not associated with sustained weight gain.
MeSH Terms
Figure
Reference
-
References
1. Wartofsky L, Van Nostrand D. Radioiodine treatment of well-differentiated thyroid cancer. Endocrine. 2012; 42(3):506–13.
Article2. Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007; 48(6):879–88.
Article3. Sohn SY, Joung JY, Cho YY, Park SM, Jin SM, Chung JH, et al. Weight changes in patients with differentiated thyroid carcinoma during postoperative longterm follow-up under thyroid stimulating hormone suppression. Endocrinol Metab (Seoul). 2015; 30(3):343–51.
Article4. Yoo ID, Kim SH, Seo YY, Oh JK, O JH, Chung SK. The success rate of initial (131)i ablation in differentiated thyroid cancer: comparison between less strict and very strict low iodine diets. Nucl Med Mol Imaging. 2012; 46(1):34–40.
Article5. Goh VH, Tain CF, Tong TY, Mok HP, Wong MT. Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. J Lipid Res. 2004; 45(10):1892–8.
Article6. Virdis A, Neves MF, Duranti E, Bernini G, Taddei S. Microvascular endothelial dysfunction in obesity and hypertension. Curr Pharm Des. 2013; 19(13):2382–9.
Article7. Forte V, Pandey A, Abdelmessih R, Forte G, Whaley-Connell A, Sowers JR, et al. Obesity, diabetes, the cardiorenal syndrome, and risk for cancer. Cardiorenal Med. 2012; 2(2):143–62.
Article8. Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008; 123(8):18926.
Article9. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011; 378(9793):81525.
Article10. Fagour C, Gonzalez C, Pezzino S, Florenty S, Rosette-Narece M, Gin H, et al. Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes Metab. 2013; 39(1):85–7.
Article11. Haslam DW, James WP. Obesity. Lancet. 2005; 366(9492):1197–209.
Article12. Rathi MS, Miles JN, Jennings PE. Weight gain during the treatment of thyrotoxicosis using conventional thyrostatic treatment. J Endocrinol Invest. 2008; 31(6):505–8.
Article13. Brunova J, Bruna J, Joubert G, Koning M. Weight gain in patients after therapy for hyperthyroidism. S Afr Med J. 2003; 93(7):529–31.14. Pears J, Jung RT, Gunn A. Long-term weight changes in treated hyperthyroid and hypothyroid patients. Scott Med J. 1990; 35(6):180–2.
Article15. Watts MR, Moore A, Alexander WD. Weight gain and treatment for thyrotoxicosis. QJM. 2002; 95(1):57–8.
Article16. Jonklaas J, Nsouli-Maktabi H. Weight changes in euthyroid patients undergoing thyroidectomy. Thyroid. 2011; 21(12):134351.
Article17. Giuffrida FM, Guedes AD, Rocco ER, Mory DB, Dualib P, Matos OS, et al. Heterogeneous behavior of lipids according to HbA1c levels undermines the plausibility of metabolic syndrome in type 1 diabetes: data from a nationwide multicenter survey. Cardiovasc Diabetol. 2012; 11:156.
Article18. Sorisky A, Bell A, Gagnon A. TSH receptor in adipose cells. Horm Metab Res. 2000; 32(11–12):46874.
Article19. Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ, Sorisky A. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol. 2000; 279(2):C335–40.
Article20. Murakami M, Kamiya Y, Morimura T, Araki O, Imamura M, Ogiwara T, et al. Thyrotropin receptors in brown adipose tissue: thyrotropin stimulates type II iodothyronine deiodinase and uncoupling protein-1 in brown adipocytes. Endocrinology. 2001; 142(3):1195–201.21. Davies T, Marians R, Latif R. The TSH receptor reveals itself. J Clin Invest. 2002; 110(2):161–4.
Article22. Lu S, Guan Q, Liu Y, Wang H, Xu W, Li X, et al. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 2012; 11:17.
Article23. Ambrosi B, Masserini B, Iorio L, Delnevo A, Malavazos AE, Morricone L, et al. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J Endocrinol Invest. 2010; 33(9):640–3.
Article24. de Lloyd A, Bursell J, Gregory JW, Rees DA, Ludgate M. TSH receptor activation and body composition. J Endocrinol. 2010; 204(1):13–20.
Article25. Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999; 84(7):): 2557–62.
Article26. Crisp M, Starkey KJ, Lane C, Ham J, Ludgate M. Adipogenesis in thyroid eye disease. Invest Ophthalmol Vis Sci. 2000; 41(11):3249–55.27. Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotrophin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003; 30(3):369–80.
Article28. Arora S. Anubhuti. Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides. 2006; 40(6):375401.29. Valcavi R, Zini M, Peino R, Casanueva FF, Dieguez C. Influence of thyroid status on serum immunoreactive leptin levels. J Clin Endocrinol Metab. 1997; 82(5):16324.
Article30. Kapadia KB, Bhatt PA, Shah JS. Association between altered thyroid state and insulin resistance. J Pharmacol Pharmacother. 2012; 3(2):156–60.31. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004; 101(13):467984.
Article32. Gimenez-Palop O, Gimenez-Perez G, Mauricio D, Berlanga E, Potau N, Vilardell C, et al. Circulating ghrelin in thyroid dysfunction is related to insulin resistance and not to hunger, food intake or anthropometric changes. Eur J Endocrinol. 2005; 153(1):73–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Dose versus High Dose Radioiodine Therapy
- Weight change in patients with differentiated thyroid carcinoma after total thyroidectomy versus lobectomy
- Surgical Methods for Radioiodine Refractory Thyroid Cancer
- Recent Advances in Radioiodine Therapy for Thyroid Cancer
- Cognitive Function of Thyroid Papillary Carcinoma Patients Before Radioiodine Therapy