Ann Dermatol.
2016 Jun;28(3):327-334. 10.5021/ad.2016.28.3.327.
Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5α-Dihydrotestosterone-Treated Human Dermal Papilla Cells
- Affiliations
-
- 1Korea Institute for Skin and Clinical Sciences and Molecular-Targeted Drug Research Center, Konkuk University, Seoul, Korea. hjcha@konkuk.ac.kr
- 2Department of Dermatology, Konkuk University School of Medicine, Seoul, Korea.
- 3KISCS Incorporated, Cheongju, Korea.
- KMID: 2164640
- DOI: http://doi.org/10.5021/ad.2016.28.3.327
Abstract
- BACKGROUND
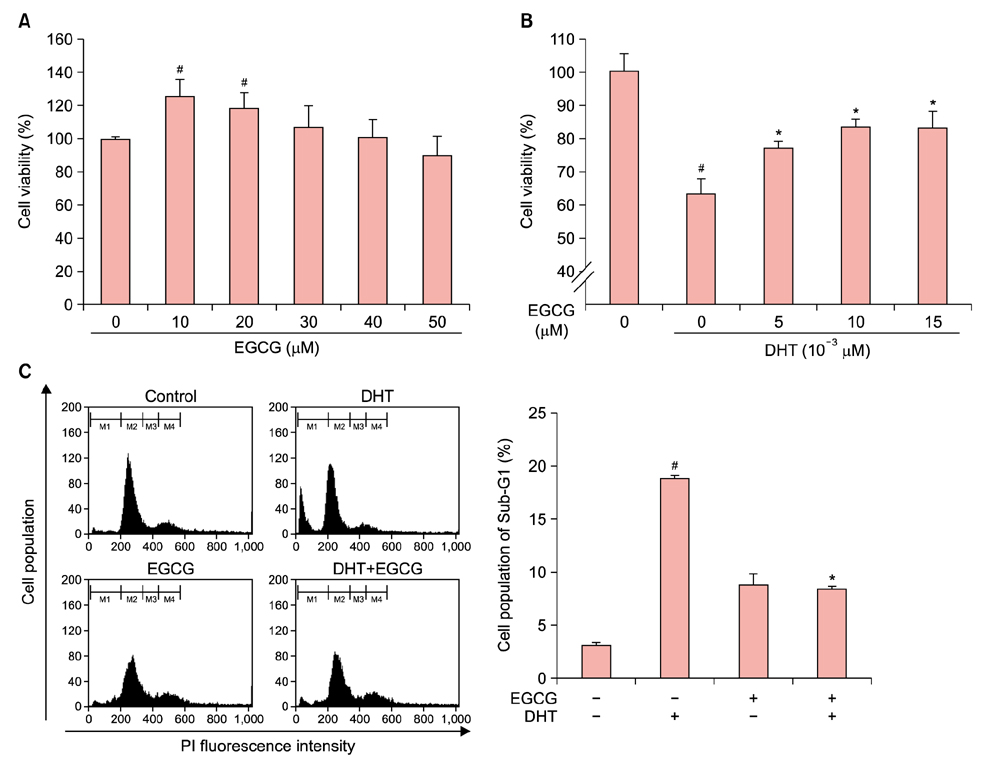
Dihydrotestosterone (DHT) induces androgenic alopecia by shortening the hair follicle growth phase, resulting in hair loss. We previously demonstrated how changes in the microRNA (miRNA) expression profile influenced DHT-mediated cell death, cell cycle arrest, cell viability, the generation of reactive oxygen species (ROS), and senescence. Protective effects against DHT have not, however, been elucidated at the genome level.
OBJECTIVE
We showed that epigallocatechin gallate (EGCG), a major component of green tea, protects DHT-induced cell death by regulating the cellular miRNA expression profile.
METHODS
We used a miRNA microarray to identify miRNA expression levels in human dermal papilla cells (DPCs). We investigated whether the miRNA expression influenced the protective effects of EGCG against DHT-induced cell death, growth arrest, intracellular ROS levels, and senescence.
RESULTS
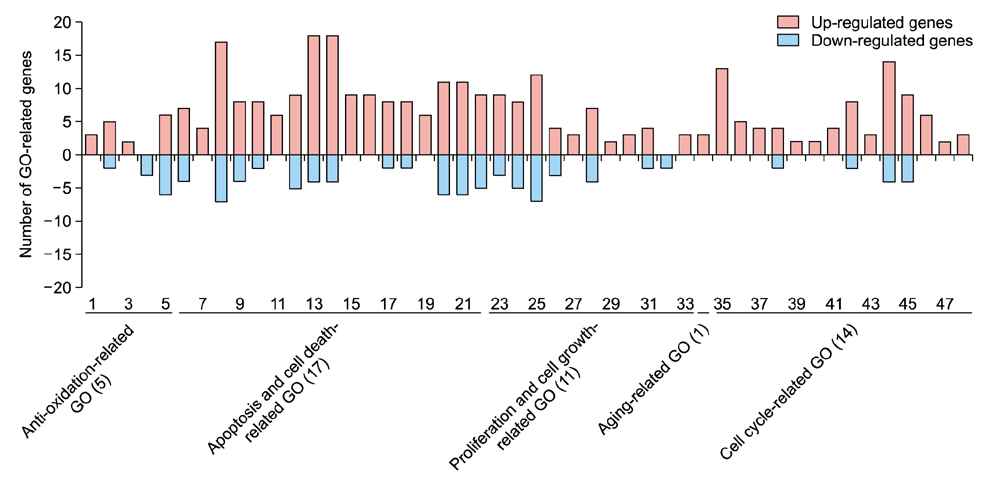
EGCG protected against the effects of DHT by altering the miRNA expression profile in human DPCs. In addition, EGCG attenuated DHT-mediated cell death and growth arrest and decreased intracellular ROS levels and senescence. A bioinformatics analysis elucidated the relationship between the altered miRNA expression and EGCG-mediated protective effects against DHT.
CONCLUSION
Overall, our results suggest that EGCG ameliorates the negative effects of DHT by altering the miRNA expression profile in human DPCs.
MeSH Terms
Figure
Reference
-
1. Van Neste D, Fuh V, Sanchez-Pedreno P, Lopez-Bran E, Wolff H, Whiting D, et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000; 143:804–810.
Article2. Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002; 37:981–990.
Article3. Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012; 24:243–252.
Article4. Hibino T, Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004; 35:9–18.5. Randall VA. Role of 5 alpha-reductase in health and disease. Baillieres Clin Endocrinol Metab. 1994; 8:405–431.6. Horton R, Pasupuletti V, Antonipillai I. Androgen induction of steroid 5 alpha-reductase may be mediated via insulinlike growth factor-I. Endocrinology. 1993; 133:447–451.
Article7. Rebora A. Pathogenesis of androgenetic alopecia. J Am Acad Dermatol. 2004; 50:777–779.
Article8. Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002; 4:1–11.
Article9. Jain R, De-Eknamkul W. Potential targets in the discovery of new hair growth promoters for androgenic alopecia. Expert Opin Ther Targets. 2014; 18:787–806.
Article10. Karnik P, Shah S, Dvorkin-Wininger Y, Oshtory S, Mirmirani P. Microarray analysis of androgenetic and senescent alopecia: comparison of gene expression shows two distinct profiles. J Dermatol Sci. 2013; 72:183–186.
Article11. Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res. 2012; 304:499–510.
Article12. Lee MJ, Cha HJ, Lim KM, Lee OK, Bae S, Kim CH, et al. Analysis of the microRNA expression profile of normal human dermal papilla cells treated with 5α-dihydrotestosterone. Mol Med Rep. 2015; 12:1205–1212.
Article13. Goodarzi HR, Abbasi A, Saffari M, Fazelzadeh Haghighi M, Tabei MB, Noori Daloii MR. Differential expression analysis of balding and nonbalding dermal papilla microRNAs in male pattern baldness with a microRNA amplification profiling method. Br J Dermatol. 2012; 166:1010–1016.
Article14. Cha HJ, Kim OY, Lee GT, Lee KS, Lee JH, Park IC, et al. Identification of ultraviolet B radiation-nduced microRNAs in normal human dermal papilla cells. Mol Med Rep. 2014; 10:1663–1670.
Article15. Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013; 32:894–903.
Article16. Shankar S, Ganapathy S, Srivastava RK. Green tea polyphenols: biology and therapeutic implications in cancer. Front Biosci. 2007; 12:4881–4899.
Article17. Du GJ, Zhang Z, Wen XD, Yu C, Calway T, Yuan CS, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012; 4:1679–1691.
Article18. Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010; 302:5–17.
Article19. Zhu W, Xu J, Ge Y, Cao H, Ge X, Luo J, et al. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J Radiat Res. 2014; 55:1056–1065.
Article20. Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001; 22:287–294.
Article21. Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structureactivity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 2002; 63:1165–1176.
Article22. Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC, et al. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. 2007; 14:551–555.
Article23. Li Y, Kong D, Wang Z, Sarkar FH. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res. 2010; 27:1027–1041.
Article24. Kim OY, Cha HJ, Ahn KJ, An IS, An S, Bae S. Identification of microRNAs involved in growth arrest and cell death in hydrogen peroxide-treated human dermal papilla cells. Mol Med Rep. 2014; 10:145–154.
Article25. Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, et al. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003; 17:1913–1915.26. Zheng Y, Lim EJ, Wang L, Smart EJ, Toborek M, Hennig B. Role of caveolin-1 in EGCG-mediated protection against linoleic-acid-induced endothelial cell activation. J Nutr Biochem. 2009; 20:202–209.
Article27. Elbling L, Herbacek I, Weiss RM, Jantschitsch C, Micksche M, Gerner C, et al. Hydrogen peroxide mediates EGCGinduced antioxidant protection in human keratinocytes. Free Radic Biol Med. 2010; 49:1444–1452.
Article28. Gu JW, Makey KL, Tucker KB, Chinchar E, Mao X, Pei I, et al. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc Cell. 2013; 5:9.
Article29. Shankar S, Marsh L, Srivastava RK. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol Cell Biochem. 2013; 372:83–94.
Article30. Fang CY, Wu CC, Hsu HY, Chuang HY, Huang SY, Tsai CH, et al. EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int J Mol Sci. 2015; 16:2530–2558.
Article31. Ahn WS, Huh SW, Bae SM, Lee IP, Lee JM, Namkoong SE, et al. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G(1) arrest, and regulation of gene expression. DNA Cell Biol. 2003; 22:217–224.
Article32. Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol. 2015; 135:1244–1252.
Article33. Liu S, Navarro G, Mauvais-Jarvis F. Androgen excess produces systemic oxidative stress and predisposes to beta-cell failure in female mice. PLoS One. 2010; 5:e11302.34. Lu LY, Ou N, Lu QB. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci Rep. 2013; 3:3169.
Article35. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011; 194:7–15.
Article36. Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, et al. MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem. 2011; 286:420–428.
Article37. Yang W, Sun T, Cao J, Liu F, Tian Y, Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res. 2012; 318:944–954.
Article38. Kiga K, Mimuro H, Suzuki M, Shinozaki-Ushiku A, Kobayashi T, Sanada T, et al. Epigenetic silencing of miR-210 increases the proliferation of gastric epithelium during chronic Helicobacter pylori infection. Nat Commun. 2014; 5:4497.
Article39. Kim JH, Park SG, Song SY, Kim JK, Sung JH. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013; 4:e588.
Article40. Jiang X, Xiang G, Wang Y, Zhang L, Yang X, Cao L, et al. MicroRNA-590-5p regulates proliferation and invasion in human hepatocellular carcinoma cells by targeting TGF-β RII. Mol Cells. 2012; 33:545–551.
Article41. Shan X, Miao Y, Fan R, Qian H, Chen P, Liu H, et al. MiR-590-5P inhibits growth of HepG2 cells via decrease of S100A10 expression and Inhibition of the Wnt pathway. Int J Mol Sci. 2013; 14:8556–8569.
Article42. Chu Y, Ouyang Y, Wang F, Zheng A, Bai L, Han L, et al. MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J Cell Biochem. 2014; 115:847–853.
Article43. Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao G, et al. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013; 67:521–526.
Article44. Wu Z, Sun H, Zeng W, He J, Mao X. Upregulation of MircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One. 2012; 7:e45825.
Article45. Wu J, Lv Q, He J, Zhang H, Mei X, Cui K, et al. MicroRNA-188 suppresses G1/S transition by targeting multiple cyclin/ CDK complexes. Cell Commun Signal. 2014; 12:66.
Article46. Schneider C, Setty M, Holmes AB, Maute RL, Leslie CS, Mussolin L, et al. MicroRNA 28 controls cell proliferation and is down-regulated in B-cell lymphomas. Proc Natl Acad Sci U S A. 2014; 111:8185–8190.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE-ORGA, a Korean Herb Extract, Can Prevent Hair Loss Induced by Dihydrotestosterone in Human Dermal Papilla Cells
- Molecular Cloning of hSC2 Encoding 5α-reductase-like Protein
- Effects of Alopecia Areata Serum on Proliferation of Cultured Dermal Papilla Cells
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- Epigallocatechin-3-gallate Regulates Inducible Nitric Oxide Synthase Expression in Human Umbilical Vein Endothelial Cells