Ann Dermatol.
2016 Jun;28(3):279-289. 10.5021/ad.2016.28.3.279.
Skin Pigmentation and Pigmentary Disorders: Focus on Epidermal/Dermal Cross-Talk
- Affiliations
-
- 1Cutaneous Physiopathology and Integrated Center of Metabolomics Research, San Gallicano Dermatologic Institute, IRCCS, Rome, Italy. picardo@ifo.it
- KMID: 2164634
- DOI: http://doi.org/10.5021/ad.2016.28.3.279
Abstract
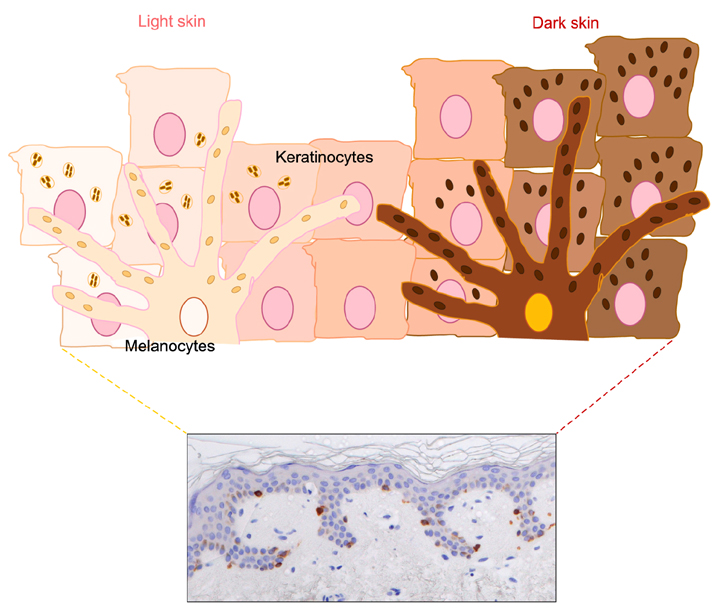
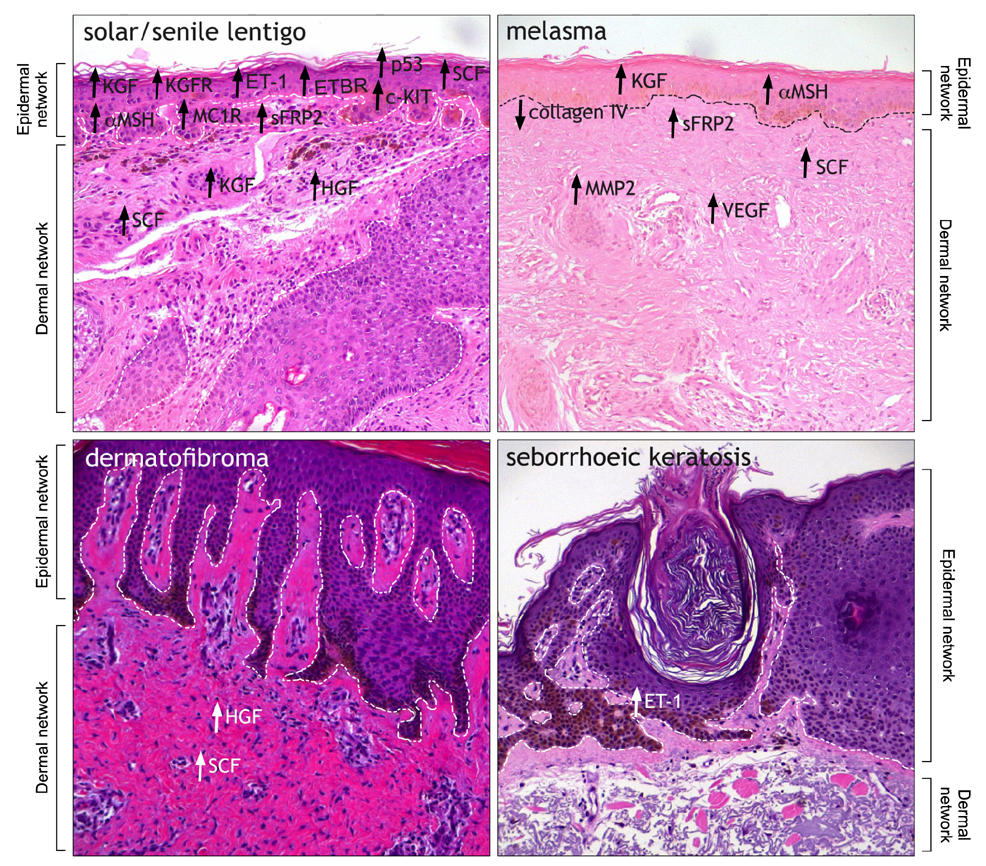
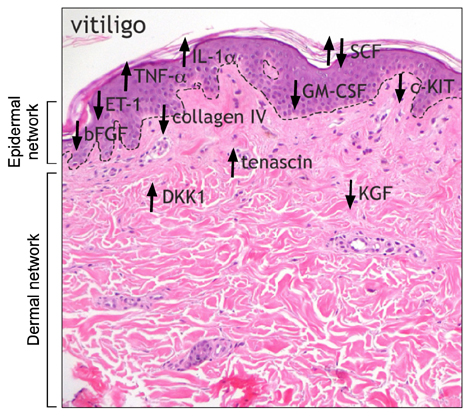
- Variation in human skin and hair color is the most notable aspect of human variability and several studies in evolution, genetics and developmental biology contributed to explain the mechanisms underlying human skin pigmentation, which is responsible for differences in skin color across the world's populations. Despite skin pigmentation is primarily related to melanocytes functionality, the surrounding keratinocytes and extracellular matrix proteins and fibroblasts in the underlying dermal compartment actively contribute to cutaneous homeostasis. Many autocrine/paracrine secreted factors and cell adhesion mechanisms involving both epidermal and dermal constituents determine constitutive skin pigmentation and, whenever deregulated, the occurrence of pigmentary disorders. In particular, an increased expression of such mediators and their specific receptors frequently lead to hyperpigmentary conditions, such as in melasma and in solar lentigo, whereas a defect in their expression/release is related to hypopigmented disorders, as seen in vitiligo. All these interactions underline the relevant role of pigmentation on human evolution and biology.
MeSH Terms
-
Biology
Cell Adhesion
Developmental Biology
Extracellular Matrix Proteins
Fibroblasts
Genetics
Hair Color
Homeostasis
Humans
Intercellular Signaling Peptides and Proteins
Keratinocytes
Lentigo
Melanocytes
Melanosis
Pigmentation
Skin Pigmentation*
Skin*
Vitiligo
Extracellular Matrix Proteins
Intercellular Signaling Peptides and Proteins
Figure
Reference
-
1. Fitzpatrick TB, Breathnach AS. The epidermal melanin unit system. Dermatol Wochenschr. 1963; 147:481–489.2. Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009; 35:193–199.
Article3. Staricco RJ, Pinkus H. Quantitative and qualitative data on the pigment cells of adult human epidermis. J Invest Dermatol. 1957; 28:33–45.
Article4. Alaluf S, Barrett K, Blount M, Carter N. Ethnic variation in tyrosinase and TYRP1 expression in photoexposed and photoprotected human skin. Pigment Cell Res. 2003; 16:35–42.
Article5. Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002; 15:112–118.
Article6. Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006; 28:79–93.
Article7. Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte vs the sun: who will win the final round? Pigment Cell Res. 2003; 16:434–447.
Article8. Smith SS, Jordan WD. An essay on the causes of the variety of complexion and figure in the human species. Cambridge: Belknap Press of Harvard University Press;1965.9. Mitchell J, Collinson P. An essay upon the causes of the different colours of people in different climates. Phil Trans. 1744; 43:102–150.10. Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000; 39:57–106.
Article11. Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009; 18:R9–R17.
Article12. Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007; 39:1443–1452.
Article13. Han J, Kraft P, Nan H, Guo Q, Chen C, Qureshi A, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008; 4:e1000074.
Article14. Rees JL. The melanocortin 1 receptor (MC1R): more than just red hair. Pigment Cell Res. 2000; 13:135–140.
Article15. Böhm M, Luger TA, Tobin DJ, García-Borrón JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006; 126:1966–1975.
Article16. Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, et al. Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol. 1998; 111:119–122.
Article17. John PR, Makova K, Li WH, Jenkins T, Ramsay M. DNA polymorphism and selection at the melanocortin-1 receptor gene in normally pigmented southern African individuals. Ann N Y Acad Sci. 2003; 994:299–306.
Article18. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007; 128:853–864.
Article19. Tada A, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, et al. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998; 9:575–584.20. Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004; 17:96–110.
Article21. Murase D, Hachiya A, Amano Y, Ohuchi A, Kitahara T, Takema Y. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J Biol Chem. 2009; 284:4343–4353.
Article22. Tomita Y, Maeda K, Tagami H. Melanocyte-stimulating properties of arachidonic acid metabolites: possible role in postinflammatory pigmentation. Pigment Cell Res. 1992; 5:357–361.
Article23. Kovacs D, Flori E, Maresca V, Ottaviani M, Aspite N, Dell'Anna ML, et al. The eumelanin intermediate 5,6-dihydroxyindole-2-carboxylic acid is a messenger in the cross-talk among epidermal cells. J Invest Dermatol. 2012; 132:1196–1205.
Article24. Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, et al. A -defensin mutation causes black coat color in domestic dogs. Science. 2007; 318:1418–1423.
Article25. Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, et al. Defining MC1R regulation in human melanocytes by its agonist α-melanocortin and antagonists agouti signaling protein and β-defensin 3. J Invest Dermatol. 2012; 132:2255–2262.
Article26. Jarrett SG, Wolf Horrell EM, Boulanger MC, D'Orazio JA. Defining the contribution of MC1R physiological ligands to ATR phosphorylation at Ser435, a predictor of DNA repair in melanocytes. J Invest Dermatol. 2015; 135:3086–3095.
Article27. Hedley S, Gawkrodger DJ, Weetman AP, MacNeil S. Investigation of the influence of extracellular matrix proteins on normal human melanocyte morphology and melanogenic activity. Br J Dermatol. 1996; 135:888–897.
Article28. Hara M, Yaar M, Tang A, Eller MS, Reenstra W, Gilchrest BA. Role of integrins in melanocyte attachment and dendricity. J Cell Sci. 1994; 107:2739–2748.
Article29. Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, et al. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci. 2010; 123:3102–3111.
Article30. Yamaguchi Y, Morita A, Maeda A, Hearing VJ. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J Investig Dermatol Symp Proc. 2009; 14:73–75.
Article31. Cardinali G, Ceccarelli S, Kovacs D, Aspite N, Lotti LV, Torrisi MR, et al. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J Invest Dermatol. 2005; 125:1190–1199.
Article32. Cardinali G, Bolasco G, Aspite N, Lucania G, Lotti LV, Torrisi MR, et al. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J Invest Dermatol. 2008; 128:558–567.
Article33. Kovacs D, Cardinali G, Aspite N, Cota C, Luzi F, Bellei B, et al. Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br J Dermatol. 2010; 163:1020–1027.
Article34. Chen N, Hu Y, Li WH, Eisinger M, Seiberg M, Lin CB. The role of keratinocyte growth factor in melanogenesis: a possible mechanism for the initiation of solar lentigines. Exp Dermatol. 2010; 19:865–872.
Article35. Snell RS. The pigmentary changes occurring in the breast skin during pregnancy and following estrogen treatment. J Invest Dermatol. 1964; 43:181–186.
Article36. Wilson MJ, Spaziani E. The melanogenic response to testosterone in scrotal epidermis: effects on tyrosinase activity and protein synthesis. Acta Endocrinol (Copenh). 1976; 81:435–448.
Article37. Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007; 29:3–14.
Article38. Si J, Lee S, Park JM, Sung J, Ko G. Genetic associations and shared environmental effects on the skin microbiome of Korean twins. BMC Genomics. 2015; 16:992–1002.
Article39. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991; 24:1–26.
Article40. Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis. J Immunol. 2010; 184:5263–5270.
Article41. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006; 203:2271–2279.
Article42. Gläser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schröder JM, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009; 123:1117–1123.
Article43. Elias PM, Menon G, Wetzel BK, Williams J. Barrier requirements as the evolutionary "driver" of epidermal pigmentation in humans. Am J Hum Biol. 2010; 22:526–537.
Article44. Jung SE, Kang HY, Lee ES, Kim YC. Changes of epidermal thickness in vitiligo. Am J Dermatopathol. 2015; 37:289–292.
Article45. Ganju P, Nagpal S, Mohammed MH, Nishal Kumar P, Pandey R, Natarajan VT, et al. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci Rep. 2016; 6:18761.
Article46. Maresca V, Flori E, Camera E, Bellei B, Aspite N, Ludovici M, et al. Linking αMSH with PPARγ in B16-F10 melanoma. Pigment Cell Melanoma Res. 2013; 26:113–127.
Article47. Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000; 13:60–69.
Article48. Herraiz C, Journé F, Abdel-Malek Z, Ghanem G, Jiménez-Cervantes C, García-Borrón JC. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol Endocrinol. 2011; 25:138–156.
Article49. Bellei B, Pitisci A, Izzo E, Picardo M. Inhibition of melanogenesis by the pyridinyl imidazole class of compounds: possible involvement of the Wnt/β-catenin signaling pathway. PLoS One. 2012; 7:e33021.
Article50. Kang HY, Chung E, Lee M, Cho Y, Kang WH. Expression and function of peroxisome proliferator-activated receptors in human melanocytes. Br J Dermatol. 2004; 150:462–468.
Article51. Flori E, Mastrofrancesco A, Kovacs D, Ramot Y, Briganti S, Bellei B, et al. 2,4,6-Octatrienoic acid is a novel promoter of melanogenesis and antioxidant defence in normal human melanocytes via PPAR-γ activation. Pigment Cell Melanoma Res. 2011; 24:618–630.
Article52. Aoki H, Moro O, Tagami H, Kishimoto J. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br J Dermatol. 2007; 156:1214–1223.
Article53. Lin CB, Hu Y, Rossetti D, Chen N, David C, Slominski A, et al. Immuno-histochemical evaluation of solar lentigines: The association of KGF/KGFR and other factors with lesion development. J Dermatol Sci. 2010; 59:91–97.
Article54. Salducci M, André N, Guéré C, Martin M, Fitoussi R, Vié K, et al. Factors secreted by irradiated aged fibroblasts induce solar lentigo in pigmented reconstructed epidermis. Pigment Cell Melanoma Res. 2014; 27:502–504.
Article55. Duval C, Cohen C, Chagnoleau C, Flouret V, Bourreau E, Bernerd F. Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: impact of photo-aging. PLoS One. 2014; 9:e114182.
Article56. Hasegawa K, Fujiwara R, Sato K, Shin J, Kim SJ, Kim M, et al. Possible involvement of keratinocyte growth factor in the persistence of hyperpigmentation in both human facial solar lentigines and melasma. Ann Dermatol. 2015; 27:626–629.
Article57. Iriyama S, Ono T, Aoki H, Amano S. Hyperpigmentation in human solar lentigo is promoted by heparanase-induced loss of heparan sulfate chains at the dermal-epidermal junction. J Dermatol Sci. 2011; 64:223–228.
Article58. Miot LD, Miot HA, Polettini J, Silva MG, Marques ME. Morphologic changes and the expression of alpha-melanocyte stimulating hormone and melanocortin-1 receptor in melasma lesions: a comparative study. Am J Dermatopathol. 2010; 32:676–682.
Article59. Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, et al. The dermal stem cell factor and c-kit are overexpressed in melasma. Br J Dermatol. 2006; 154:1094–1099.
Article60. Lee DJ, Park KC, Ortonne JP, Kang HY. Pendulous melanocytes: a characteristic feature of melasma and how it may occur. Br J Dermatol. 2012; 166:684–686.
Article61. Torres-Álvarez B, Mesa-Garza IG, Castanedo-Cázares JP, Fuentes-Ahumada C, Oros-Ovalle C, Navarrete-Solis J, et al. Histochemical and immunohistochemical study in melasma: evidence of damage in the basal membrane. Am J Dermatopathol. 2011; 33:291–295.
Article62. Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci. 2007; 46:111–116.
Article63. Kim M, Han JH, Kim JH, Park TJ, Kang HY. Secreted frizzled-related protein 2 (sFRP2) functions as a melanogenic stimulator; the role of sFRP2 in UV-induced hyperpigmentary disorders. J Invest Dermatol. 2016; 136:236–244.
Article64. Shishido E, Kadono S, Manaka I, Kawashima M, Imokawa G. The mechanism of epidermal hyperpigmentation in dermatofibroma is associated with stem cell factor and hepatocyte growth factor expression. J Invest Dermatol. 2001; 117:627–633.
Article65. Okazaki M, Yoshimura K, Suzuki Y, Uchida G, Kitano Y, Harii K, et al. The mechanism of epidermal hyperpigmentation in café-au-lait macules of neurofibromatosis type 1 (von Recklinghausen's disease) may be associated with dermal fibroblast-derived stem cell factor and hepatocyte growth factor. Br J Dermatol. 2003; 148:689–697.
Article66. Cardinali G, Kovacs D, Giglio MD, Cota C, Aspite N, Amantea A, et al. A kindred with familial progressive hyperpigmentation-like disorder: implication of fibroblast-derived growth factors in pigmentation. Eur J Dermatol. 2009; 19:469–473.
Article67. Lan CC, Wu CS, Cheng CM, Yu CL, Chen GS, Yu HS. Pigmentation in basal cell carcinoma involves enhanced endothelin-1 expression. Exp Dermatol. 2005; 14:528–534.
Article68. Teraki E, Tajima S, Manaka I, Kawashima M, Miyagishi M, Imokawa G. Role of endothelin-1 in hyperpigmentation in seborrhoeic keratosis. Br J Dermatol. 1996; 135:918–923.
Article69. Picardo M, Dell'Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers. 2015; 1:15011.
Article70. XXII International Pigment Cell Conference (IPCC). "Bringing Colors to Life: Advances in Pigment Cell Research and Translation into Clinical Practice" Organised by the Asian Society for Pigment Cell Research (ASPCR), in partnership with the Dermatological Society of Singapore (DSS). Pigment Cell Melanoma Res. 2014; 27:849–1001.71. Bellei B, Pitisci A, Ottaviani M, Ludovici M, Cota C, Luzi F, et al. Vitiligo: a possible model of degenerative diseases. PLoS One. 2013; 8:e59782.
Article72. Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T, et al. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol. 2004; 202:463–475.
Article73. Moretti S, Fabbri P, Baroni G, Berti S, Bani D, Berti E, et al. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009; 24:849–857.74. Lee AY, Kim NH, Choi WI, Youm YH. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J Invest Dermatol. 2005; 124:976–983.
Article75. Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002; 15:87–92.
Article76. Wagner RY, Luciani F, Cario-André M, Rubod A, Petit V, Benzekri L, et al. Altered e-cadherin levels and distribution in melanocytes precede clinical manifestations of vitiligo. J Invest Dermatol. 2015; 135:1810–1819.
Article77. Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Tenascin is overexpressed in vitiligo lesional skin and inhibits melanocyte adhesion. Br J Dermatol. 1997; 137:171–178.
Article78. Ricard AS, Pain C, Daubos A, Ezzedine K, Lamrissi-Garcia I, Bibeyran A, et al. Study of CCN3 (NOV) and DDR1 in normal melanocytes and vitiligo skin. Exp Dermatol. 2012; 21:411–416.
Article79. Oh SH, Kim JY, Kim MR, Do JE, Shin JY, Hann SK. DKK1 is highly expressed in the dermis of vitiligo lesion: is there association between DKK1 and vitiligo. J Dermatol Sci. 2012; 66:163–165.
Article80. Purpura V, Persechino F, Belleudi F, Scrofani C, Raffa S, Persechino S, et al. Decreased expression of KGF/FGF7 and its receptor in pathological hypopigmentation. J Cell Mol Med. 2014; 18:2553–2557.
Article81. Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011; 24:401–410.
Article82. Ueno M, Aoto T, Mohri Y, Yokozeki H, Nishimura EK. Coupling of the radiosensitivity of melanocyte stem cells to their dormancy during the hair cycle. Pigment Cell Melanoma Res. 2014; 27:540–551.
Article83. Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, et al. Narrow band ultraviolet B treatment for human vitiligo is associated with proliferation, migration, and differentiation of melanocyte precursors. J Invest Dermatol. 2015; 135:2068–2076.
Article84. Taïeb A, Picardo M. VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007; 20:27–35.
Article85. Esmat SM, El-Tawdy AM, Hafez GA, Zeid OA, Abdel Halim DM, Saleh MA, et al. Acral lesions of vitiligo: why are they resistant to photochemotherapy? J Eur Acad Dermatol Venereol. 2012; 26:1097–1104.
Article86. Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, et al. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2010; 123:853–860.
Article87. Okamoto N, Aoto T, Uhara H, Yamazaki S, Akutsu H, Umezawa A, et al. A melanocyte--melanoma precursor niche in sweat glands of volar skin. Pigment Cell Melanoma Res. 2014; 27:1039–1050.
Article88. Jang YH, Kim SL, Lee JS, Kwon KY, Lee SJ, Kim do W, et al. Possible existence of melanocytes or melanoblasts in human sebaceous glands. Ann Dermatol. 2014; 26:469–473.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four Cases of Pigmentary Dermacation Lines of Pregnancy with Erythema
- Two Cases of Pigmentary Demarcation Lines Associated with Pregnancy
- Two Cases of Vitiligo Developed on the Persisting Dermal Melanocytosis: Is There a Difference between Epidermal Melanocytes and Dermal Melanocytes?
- Recurrent Type B Pigmentary Demarcation Lines in Pregnancy: A Case Report
- Type B Pigmentary Demarcation Lines of Pregnancy Involving the Anterior Thighs and Knees