J Vet Sci.
2015 Jun;16(2):203-211. 10.4142/jvs.2015.16.2.203.
A study of experimental autoimmune encephalomyelitis in dogs as a disease model for canine necrotizing encephalitis
- Affiliations
-
- 1Institute of Animal Medicine, College of Veterinary Medicine, Gyeongsang National University, Jinju 660-701, Korea. jungdi@gnu.ac.kr
- 2Department of Pathobiology, Small Animal Tumor Diagnostic Center, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 3Department of Cell and Developmental Biology, DRI and Brain Korea 21 Program, School of Dentistry, Seoul National University, Seoul 110-749, Korea.
- KMID: 2164530
- DOI: http://doi.org/10.4142/jvs.2015.16.2.203
Abstract
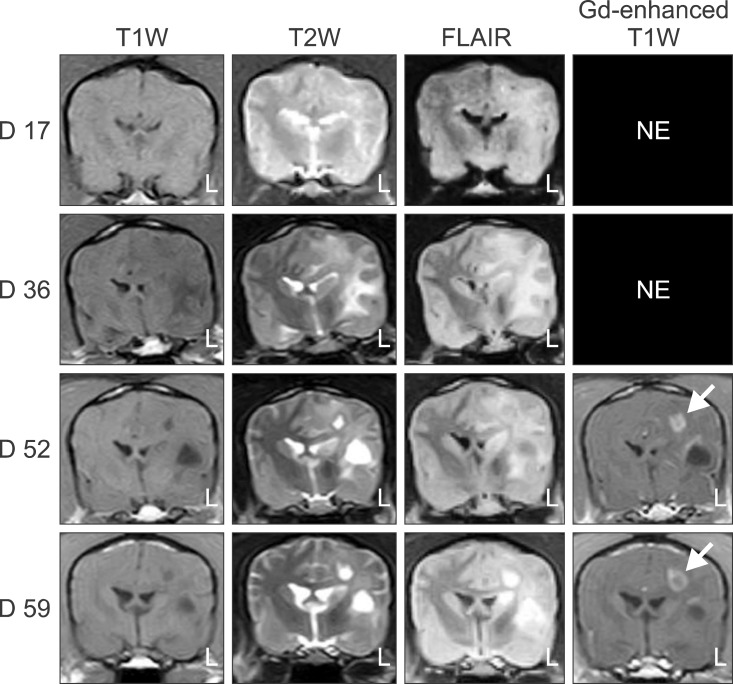
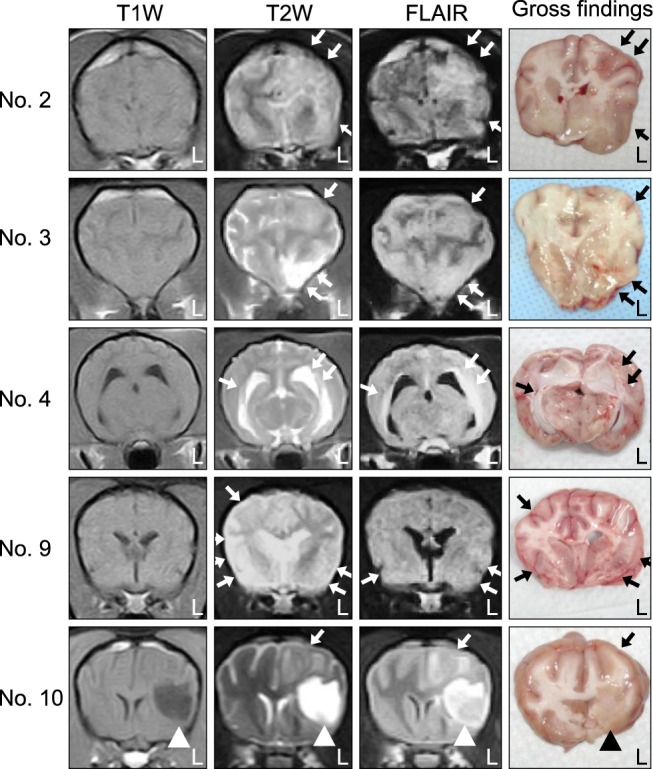
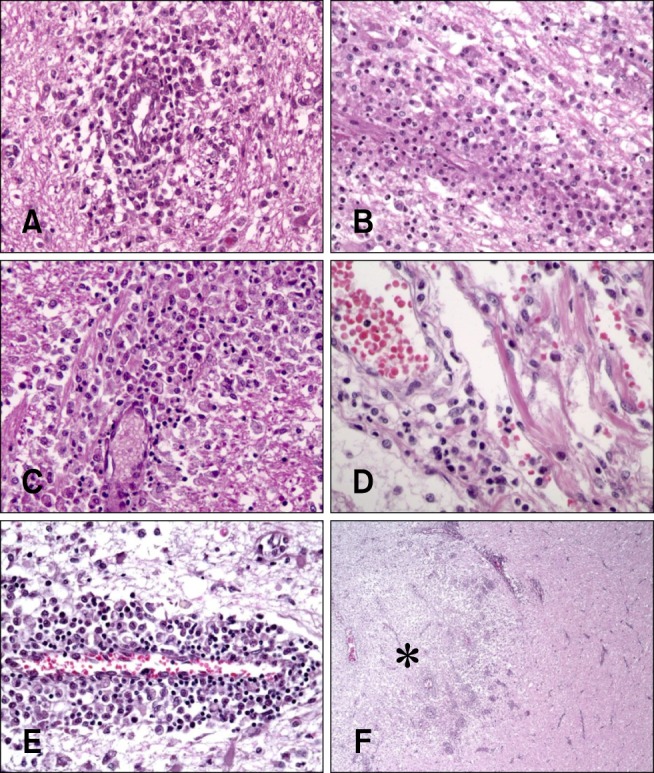
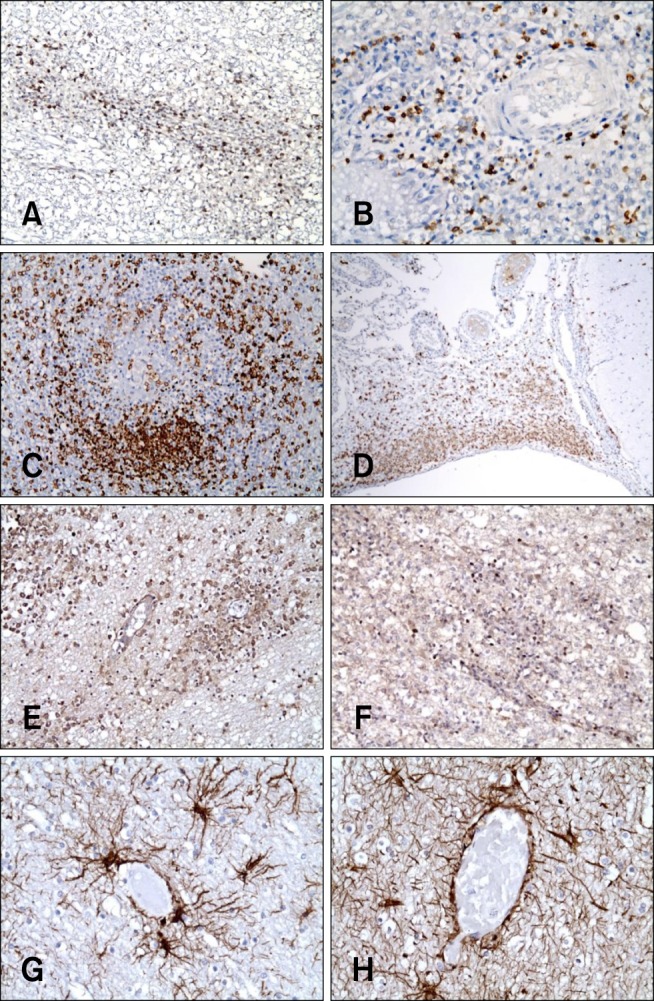
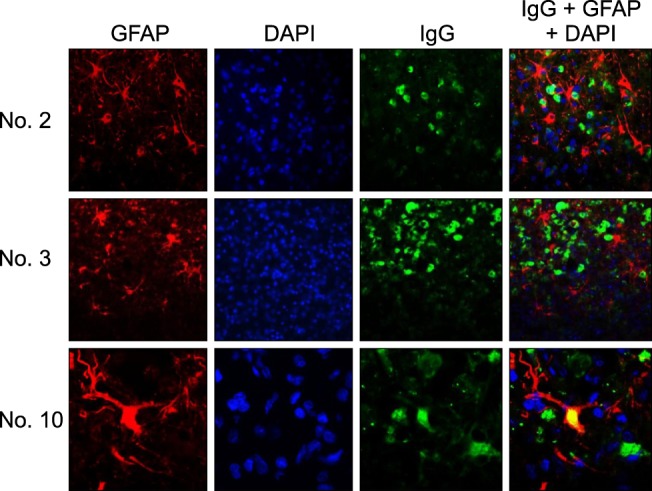
- In the present study, the use of dogs with experimental autoimmune encephalomyelitis (EAE) as a disease model for necrotizing encephalitis (NE) was assessed. Twelve healthy dogs were included in this study. Canine forebrain tissues (8 g), including white and grey matter, were homogenized with 4 mL of phosphate-buffered saline for 5 min in an ice bath. The suspension was emulsified with the same volume of Freund's complete adjuvant containing 1 mg/mL of killed Mycobacterium tuberculosis H37Ra. Under sedation, each dog was injected subcutaneously with canine brain homogenate at four sites: two in the inguinal and two in the axillary regions. A second injection (booster) was administered to all the dogs using the same procedure 7 days after the first injection. Clinical assessment, magnetic resonance imaging, cerebrospinal fluid analyses, necropsies, and histopathological and immunohistochemical examinations were performed for the dogs with EAE. Out of the 12 animals, seven (58%) developed clinically manifest EAE at various times after immunization. Characteristics of canine EAE models were very similar to canine NE, suggesting that canine EAE can be a disease model for NE in dogs.
Keyword
MeSH Terms
-
Animals
Brain/*pathology
Disease Models, Animal
Dog Diseases/*immunology
Dogs
Encephalitis/immunology/*veterinary
Encephalomyelitis, Autoimmune, Experimental/immunology/*veterinary
Female
Fluorescent Antibody Technique/veterinary
Immunization/veterinary
Immunohistochemistry/veterinary
Magnetic Resonance Imaging/veterinary
Male
Necrosis/immunology/*veterinary
Figure
Reference
-
1. Adamo PF, Adams WM, Steinberg H. Granulomatous meningoencephalomyelitis in dogs. Compend Contin Educ Vet. 2007; 29:678–690. PMID: 18210978.2. Behr S, Trumel C, Cauzinille L, Palenché F, Braun JP. High resolution protein electrophoresis of 100 paired canine cerebrospinal fluid and serum. J Vet Intern Med. 2006; 20:657–662. PMID: 16734104.
Article3. Bichsel P, Vandevelde M, Vandevelde E, Affolter U, Pfister H. Immunoelectrophoretic determination of albumin and IgG in serum and cerebrospinal fluid in dogs with neurological diseases. Res Vet Sci. 1984; 37:101–107. PMID: 6473908.
Article4. Boretius S, Schmelting B, Watanabe T, Merkler D, Tammer R, Czéh B, Michaelis T, Frahm J, Fuchs E. Monitoring of EAE onset and progression in the common marmoset monkey by sequential high-resolution 3D MRI. NMR Biomed. 2006; 19:41–49. PMID: 16408325.
Article5. Brok HP, Uccelli A, Kerlero de Rosbo N, Bontrop RE, Roccatagliata L, de Groot NG, Capello E, Laman JD, Nicolay K, Mancardi GL, Ben-Nun A, 't Hart BA. Myelin/oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in common marmosets: the encephalitogenic T cell epitope pMOG24-36 is presented by a monomorphic MHC class II molecule. J Immunol. 2000; 165:1093–1101. PMID: 10878388.
Article6. Chrisman CL. Cerebrospinal fluid analysis. Vet Clin North Am Small Anim Pract. 1992; 22:781–810. PMID: 1641918.
Article7. Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011; 164:1079–1106. PMID: 21371012.
Article8. Dousset V, Ballarino L, Delalande C, Coussemacq M, Canioni P, Petry KG, Caillé JM. Comparison of ultrasmall particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis. AJNR Am J Neuroradiol. 1999; 20:223–227. PMID: 10094342.9. Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G. Vaccination with amyloid-β peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003; 126:285–291. PMID: 12538398.10. Greer KA, Wong AK, Liu H, Famula TR, Pedersen NC, Ruhe A, Wallace M, Neff MW. Necrotizing meningoencephalitis of Pug dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens. 2010; 76:110–118. PMID: 20403140.
Article11. Hart BA, Bauer J, Muller HJ, Melchers B, Nicolay K, Brok H, Bontrop RE, Lassmann H, Massacesi L. Histopathological characterization of magnetic resonance imaging-detectable brain white matter lesions in a primate model of multiple sclerosis: a correlative study in the experimental autoimmune encephalomyelitis model in common marmosets (Callithrix jacchus). Am J Pathol. 1998; 153:649–663. PMID: 9708823.12. Hohlfeld R, Wekerle H. Immunological update on multiple sclerosis. Curr Opin Neurol. 2001; 14:299–304. PMID: 11371751.
Article13. Kipar A, Baumgärtner W, Vogl C, Gaedke K, Wellman M. Immunohistochemical characterization of inflammatory cells in brains of dogs with granulomatous meningoencephalitis. Vet Pathol. 1998; 35:43–52. PMID: 9545134.
Article14. Kojima K, Berger T, Lassmann H, Hinze-Selch D, Zhang Y, Gehrmann J, Reske K, Wekerle H, Linington C. Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100β molecule, a calcium binding protein of astroglia. J Exp Med. 1994; 180:817–829. PMID: 7520474.15. Kuharik MA, Edwards MK, Farlow MR, Becker GJ, Azzarelli B, Klatte EC, Augustyn GT, Dreesen RG. Gd-enhanced MR imaging of acute and chronic experimental demyelinating lesions. AJNR Am J Neuroradiol. 1988; 9:643–648. PMID: 3135711.16. Lassmann H, Kitz K, Wisniewski HM. Structural variability of demyelinating lesions in different models of subacute and chronic experimental allergic encephalomyelitis. Acta Neuropathol. 1980; 51:191–201. PMID: 7445973.
Article17. Levine S. Hyperacute, neutrophilic, and localized forms of experimental allergic encephalomyelitis: a review. Acta Neuropathol. 1974; 28:179–189. PMID: 4139872.
Article18. Levine S, Wenk EJ. Studies on the mechanism of altered susceptibility to experimental allergic encephalomyelitis. Am J Pathol. 1961; 39:419–441. PMID: 13761512.19. Matsuki N, Fujiwara K, Tamahara S, Uchida K, Matsunaga S, Nakayama H, Doi K, Ogawa H, Ono K. Prevalence of autoantibody in cerebrospinal fluids from dogs with various CNS diseases. J Vet Med Sci. 2004; 66:295–297. PMID: 15107560.
Article20. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001; 50:121–127. PMID: 11456302.
Article21. McRae BL, Miller SD. Fine specificity of CD4+ T cell responses to the dominant encephalitogenic PLP 139-151 peptide in SJL/J mice. Neurochem Res. 1994; 19:997–1004. PMID: 7528357.22. Park ES, Uchida K, Nakayama H. Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME). Vet Pathol. 2012; 49:682–692. PMID: 22262353.
Article23. Pellkofer H, Schubart AS, Höftberger R, Schutze N, Pagany M, Schüller M, Lassmann H, Hohlfeld R, Voltz R, Linington C. Modelling paraneoplastic CNS disease: T-cells specific for the onconeuronal antigen PNMA1 mediate autoimmune encephalomyelitis in the rat. Brain. 2004; 127:1822–1830. PMID: 15201193.
Article24. Sakai K, Zamvil SS, Mitchell DJ, Lim M, Rothbard JB, Steinman L. Characterization of a major encephalitogenic T cell epitope in SJL/J mice with synthetic oligopeptides of myelin basic protein. J Neuroimmunol. 1988; 19:21–32. PMID: 2456304.
Article25. Sorjonen DC. Total protein, albumin quota, and electrophoretic patterns in cerebrospinal fluid of dogs with central nervous system disorders. Am J Vet Res. 1987; 48:301–305. PMID: 3826872.26. Sorjonen DC, Cox NR, Swango LJ. Electrophoretic determination of albumin and gamma globulin concentrations in the cerebrospinal fluid of dogs with encephalomyelitis attributable to canine distemper virus infection: 13 cases (1980-1987). J Am Vet Med Assoc. 1989; 195:977–980. PMID: 2477352.27. Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005; 23:683–747. PMID: 15771584.
Article28. Spitzbarth I, Schenk HC, Tipold A, Beineke A. Immunohistochemical characterization of inflammatory and glial responses in a case of necrotizing leucoencephalitis in a French bulldog. J Comp Pathol. 2010; 142:235–241. PMID: 19815229.
Article29. Suzuki M, Uchida K, Morozumi M, Hasegawa T, Yanai T, Nakayama H, Tateyama S. A comparative pathology study on canine necrotizing meningoencephalitis and granulomatous meningoencephalomyelitis. J Vet Med Sci. 2003; 65:1233–1239. PMID: 14665754.30. Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract. 2010; 51:138–149. PMID: 19814766.
Article31. Thomas L, Paterson PY, Smithwick B. Acute disseminated encephalomyelitis following immunization with homologous brain extracts, I. Studies on the role of a circulating antibody in the production of the condition in dogs. J Exp Med. 1950; 92:133–152. PMID: 15428583.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Relapsed Acute Disseminated Encephalomyelitis
- Modeling of transmission pathways on canine heartworm dynamics
- Erythropoietin and autoimmune neuroinflammation: lessons from experimental autoimmune encephalomyelitis and experimental autoimmune neuritis
- Corticosteroid Treatment in Autoimmune Encephalitis
- Effects of Dexamethasone on Neurogenic Bladder in Experimental Autoimmune Encephalomyelitis Rat