J Korean Med Sci.
2015 Jul;30(7):965-973. 10.3346/jkms.2015.30.7.965.
MRI Findings and Prediction of Time to Progression of Patients with Hepatocellular Carcinoma Treated with Drug-eluting Bead Transcatheter Arterial Chemoembolization
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiological Science, Yonsei University, College of Medicine, Seoul, Korea.
- 2Department of Radiology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. bellenina@daum.net
- 3Department of Radiology and Medical Research Institute, School of Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 2164484
- DOI: http://doi.org/10.3346/jkms.2015.30.7.965
Abstract
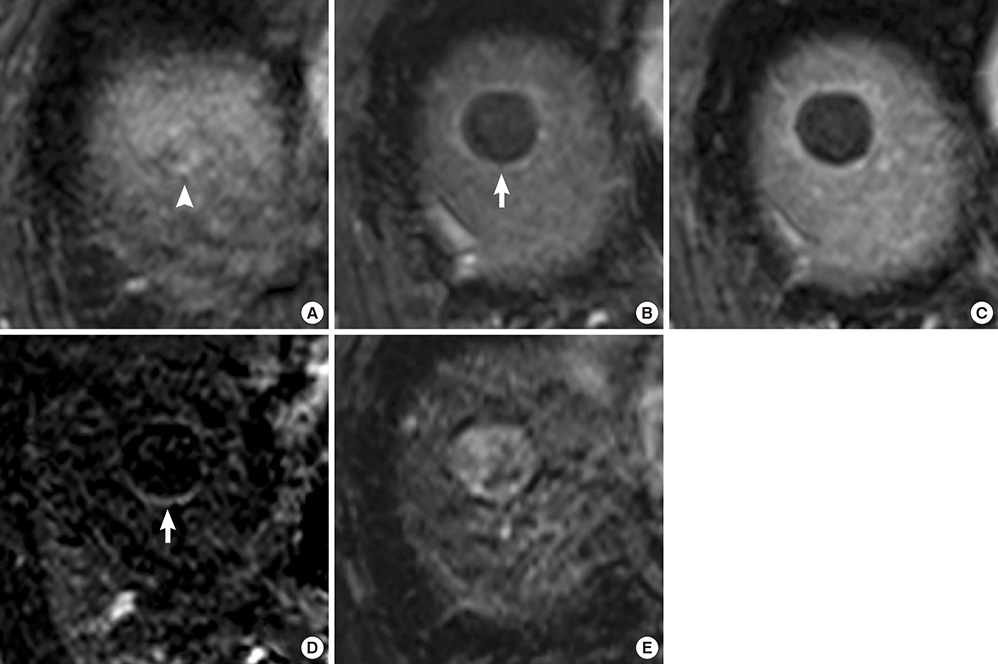
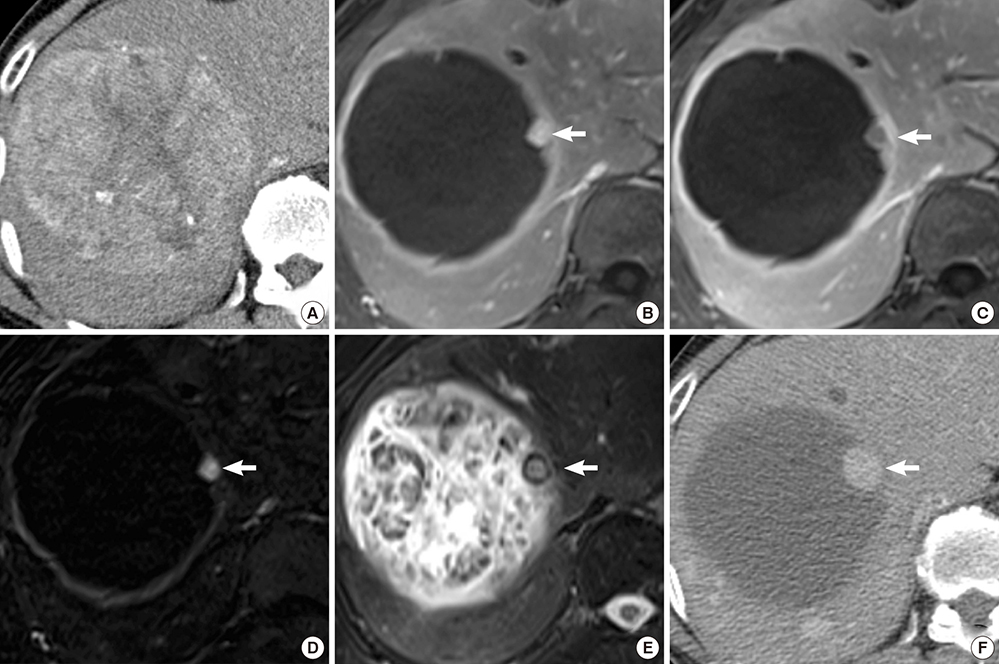
- The purpose of this study was to investigate the utility of MRI findings after drug-eluting beads (DEB) - transcatheter arterial chemoembolization (TACE) for hepatocellular carcinoma in predicting time to progression (TTP). This study included 48 patients with 60 lesions who underwent liver MRI within 3 months after DEB-TACE. MRI was assessed for arterial enhancement pattern, late washout, arterioportal shunt, signal intensity on T2-weighted image, intratumoral septa, enhancing tissue on subtraction images, and treatment response. Cox-regression analysis was performed to identify independent factors to predict TTP. TTP was calculated using the Kaplan-Meier method with the log-rank test. Per lesion, 30 achieved complete remission, 22 had a partial response, and the remaining 8 lesions displayed stable disease on MRI. Arterial enhancement pattern, washout and enhancing tissue on subtraction images from MRI were associated with viable tumor on the last follow-up computerized tomography. Arterial enhancement, washout and enhancing tissue on subtraction images were significant predictors of TTP, but only enhancing tissue on subtraction images remained a significant predictor of TTP (P=0.018) in the multivariate analysis. TTP was longer in the group without enhancing tissue on subtraction images compared to the group with enhancing tissue (601 days vs. 287 days, P<0.001). Enhancing tissue on subtraction images from MRI after DEB-TACE is predictive for longer TTP.
Keyword
MeSH Terms
-
Adult
Aged
Antineoplastic Agents/administration & dosage/*therapeutic use
Carcinoma, Hepatocellular/*drug therapy
*Chemoembolization, Therapeutic
Disease Progression
Drug Carriers/*pharmacology
Female
Humans
Liver Neoplasms/*drug therapy
Magnetic Resonance Imaging
Male
Microspheres
Middle Aged
Multidetector Computed Tomography
Treatment Outcome
Antineoplastic Agents
Drug Carriers
Figure
Reference
-
1. Kim KA, Kim MJ, Choi JY, Chung YE. Development of hepatocellular carcinomas in patients with absence of tumors on a prior ultrasound examination. Eur J Radiol. 2012; 81:1450–1454.2. Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012; 39:503–509.3. Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, Lenoir C, Attali P, Etienne JP. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990; 11:181–184.4. Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988; 94:453–456.5. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739.6. Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007; 30:6–25.7. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003; 37:429–442.8. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002; 35:1164–1171.9. Sottani C, Poggi G, Quaretti P, Regazzi M, Montagna B, Quaquarini E, Imbriani M, Leoni E, Di Cesare P, Riccardi A, et al. Serum pharmacokinetics in patients treated with transarterial chemoembolization (TACE) using two types of epirubicin-loaded microspheres. Anticancer Res. 2012; 32:1769–1774.10. Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010; 21:1084–1090.11. Lee KH, Liapi EA, Cornell C, Reb P, Buijs M, Vossen JA, Ventura VP, Geschwind JF. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol. 2010; 33:576–582.12. Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012; 57:1244–1250.13. Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011; 22:1545–1552.14. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010; 33:41–52.15. Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012; 56:1330–1335.16. Chung WS, Lee KH, Park MS, Lee YJ, Kwon J, Baek SE, Kim MJ. Enhancement patterns of hepatocellular carcinoma after transarterial chemoembolization using drug-eluting beads on arterial phase CT images: a pilot retrospective study. AJR Am J Roentgenol. 2012; 199:349–359.17. Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim DY, Ahn SH, Kim MD, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015; 62:1304–1310.18. Kamel IR, Bluemke DA. Magnetic resonance imaging of the liver: assessing response to treatment. Top Magn Reson Imaging. 2002; 13:191–200.19. Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, Charu V, Schernthaner R, Wang Z, Tacher V, et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014; 273:746–758.20. Vandecaveye V, Michielsen K, De Keyzer F, Laleman W, Komuta M, Op de beeck K, Roskams T, Nevens F, Verslype C, Maleux G. Chemoembolization for hepatocellular carcinoma: 1-month response determined with apparent diffusion coefficient is an independent predictor of outcome. Radiology. 2014; 270:747–757.21. Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011; 55:1309–1316.22. Kim BK, Kim KA, An C, Yoo EJ, Park JY, Kim DY, Ahn SH, Han KH, Kim SU, Kim MJ. Prognostic role of magnetic resonance imaging vs. computed tomography for hepatocellular carcinoma undergoing chemoembolization. Liver Int. 2015; 35:1722–1730.23. Katyal S, Oliver JH, Peterson MS, Chang PJ, Baron RL, Carr BI. Prognostic significance of arterial phase CT for prediction of response to transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: a retrospective analysis. AJR Am J Roentgenol. 2000; 175:1665–1672.24. Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, Maturen KE, Chenevert TL, Hussain HK. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013; 266:452–461.25. Minami Y, Kudo M. Therapeutic response assessment of transcatheter arterial chemoembolization for hepatocellular carcinoma: ultrasonography, CT and MR imaging. Oncology. 2013; 84:58–63.26. Ippolito D, Fior D, Bonaffini PA, Capraro C, Leni D, Corso R, Sironi S. Quantitative evaluation of CT-perfusion map as indicator of tumor response to transarterial chemoembolization and radiofrequency ablation in HCC patients. Eur J Radiol. 2014; 83:1665–1671.27. Malagari K. Drug-eluting particles in the treatment of HCC: chemoembolization with doxorubicin-loaded DC Bead. Expert Rev Anticancer Ther. 2008; 8:1643–1650.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent advance in international management of hepatocellular carcinoma
- Pirarubicin, UFT, Leucovorin Chemotherapy in Non-embolizable and Transcatheter Arterial Chemoembolization-Failed Hepatocellular Carcinoma Patients; A Phase II Clinical Study
- Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: Now and future
- The update on transcatheter arterial chemoembolization using drug-eluting beads: Optimization for best response
- A case of hepatocellular carcinoma in the caudate lobe successfully treated by transcatheter arterial chemoembolization using drug-eluting beads