Int J Stem Cells.
2016 May;9(1):53-59. 10.15283/ijsc.2016.9.1.53.
Genetically Engineered In Vitro Erythropoiesis
- Affiliations
-
- 1Department of Basic Science Research, Cellologi, LLC, USA. contact@cellologi.com
- 2Santa Barbara Cottage Hospital, Santa Barbara, California, USA.
- KMID: 2164161
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.53
Abstract
- BACKGROUND
Engineered blood has the greatest potential to combat a predicted future shortfall in the US blood supply for transfusion treatments. Engineered blood produced from hematopoietic stem cell (HSC) derived red blood cells in a laboratory is possible, but critical barriers exist to the production of clinically relevant quantities of red blood cells required to create a unit of blood. Erythroblasts have a finite expansion capacity and there are many negative regulatory mechanisms that inhibit in vitro erythropoiesis. In order to overcome these barriers and enable mass production, the expansion capacity of erythroblasts in culture will need to be exponentially improved over the current state of art. This work focused on the hypothesis that genetic engineering of HSC derived erythroblasts can overcome these obstacles.
OBJECTIVES
The objective of this research effort was to improve in vitro erythropoiesis efficiency from human adult stem cell derived erythroblasts utilizing genetic engineering. The ultimate goal is to enable the mass production of engineered blood.
METHODS
HSCs were isolated from blood samples and cultured in a liquid media containing growth factors. Cells were transfected using a Piggybac plasmid transposon.
RESULTS
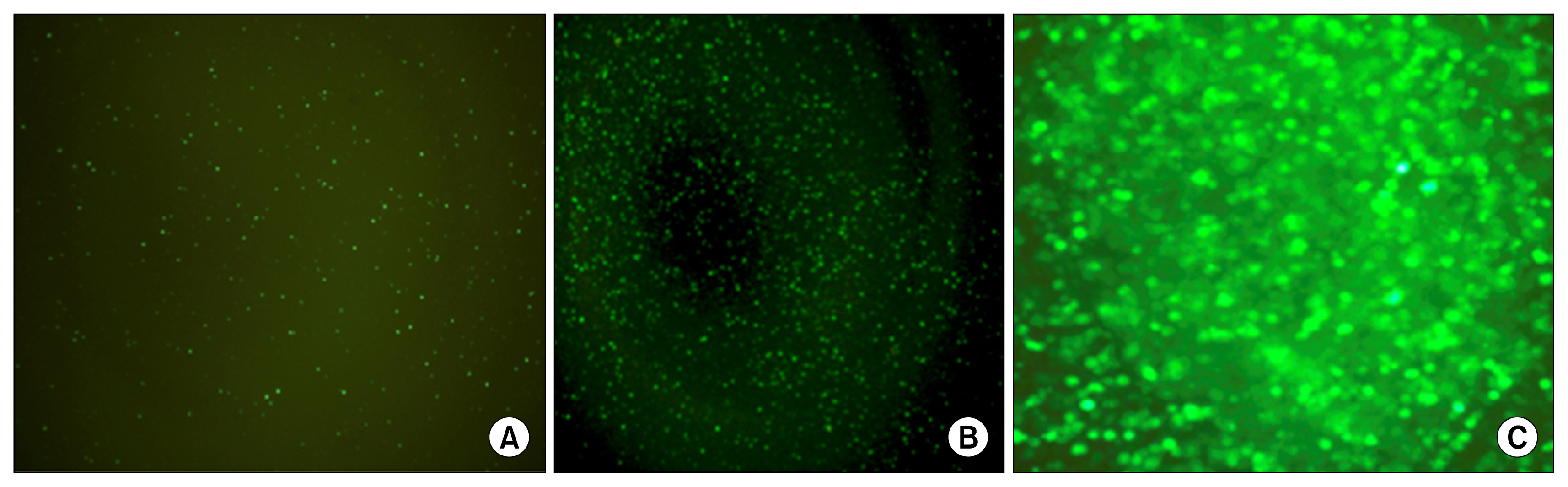
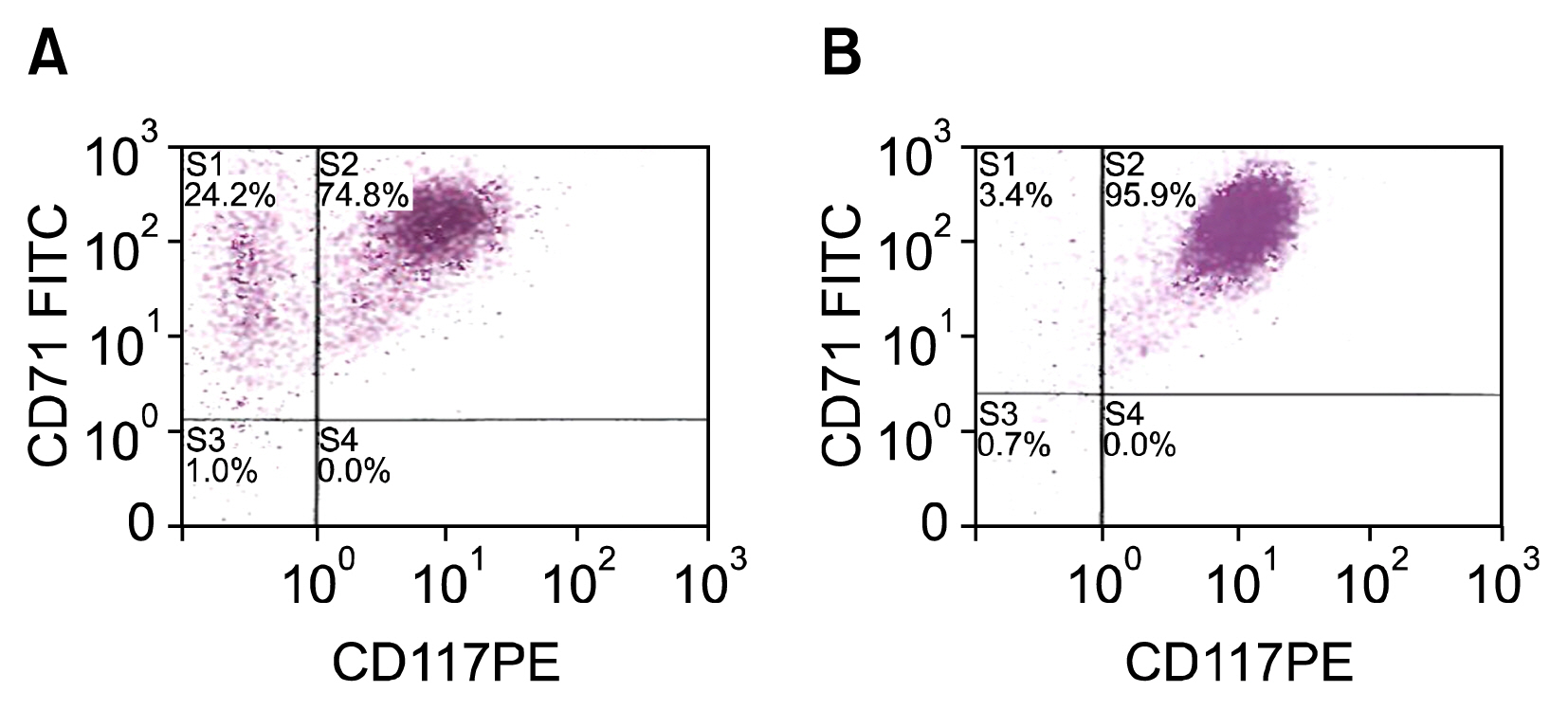
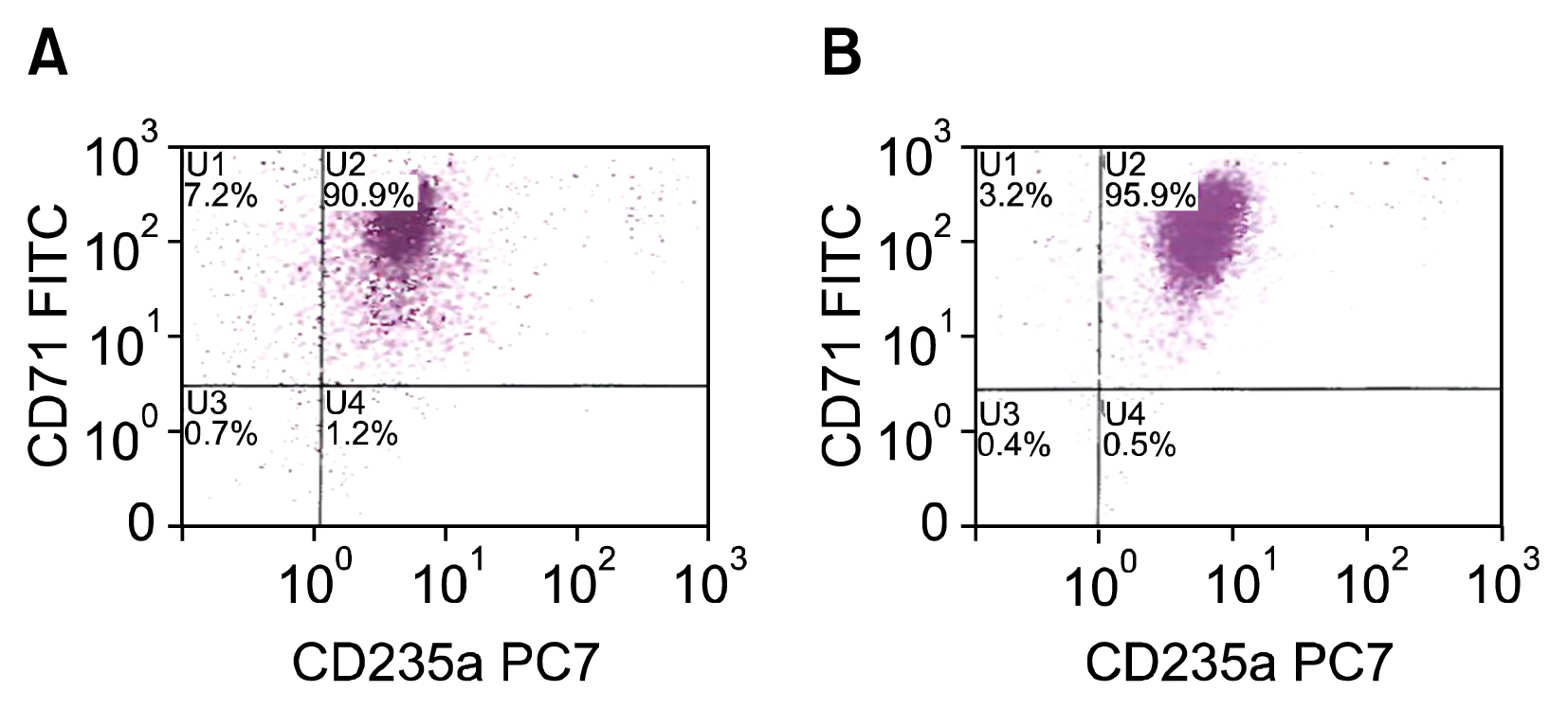
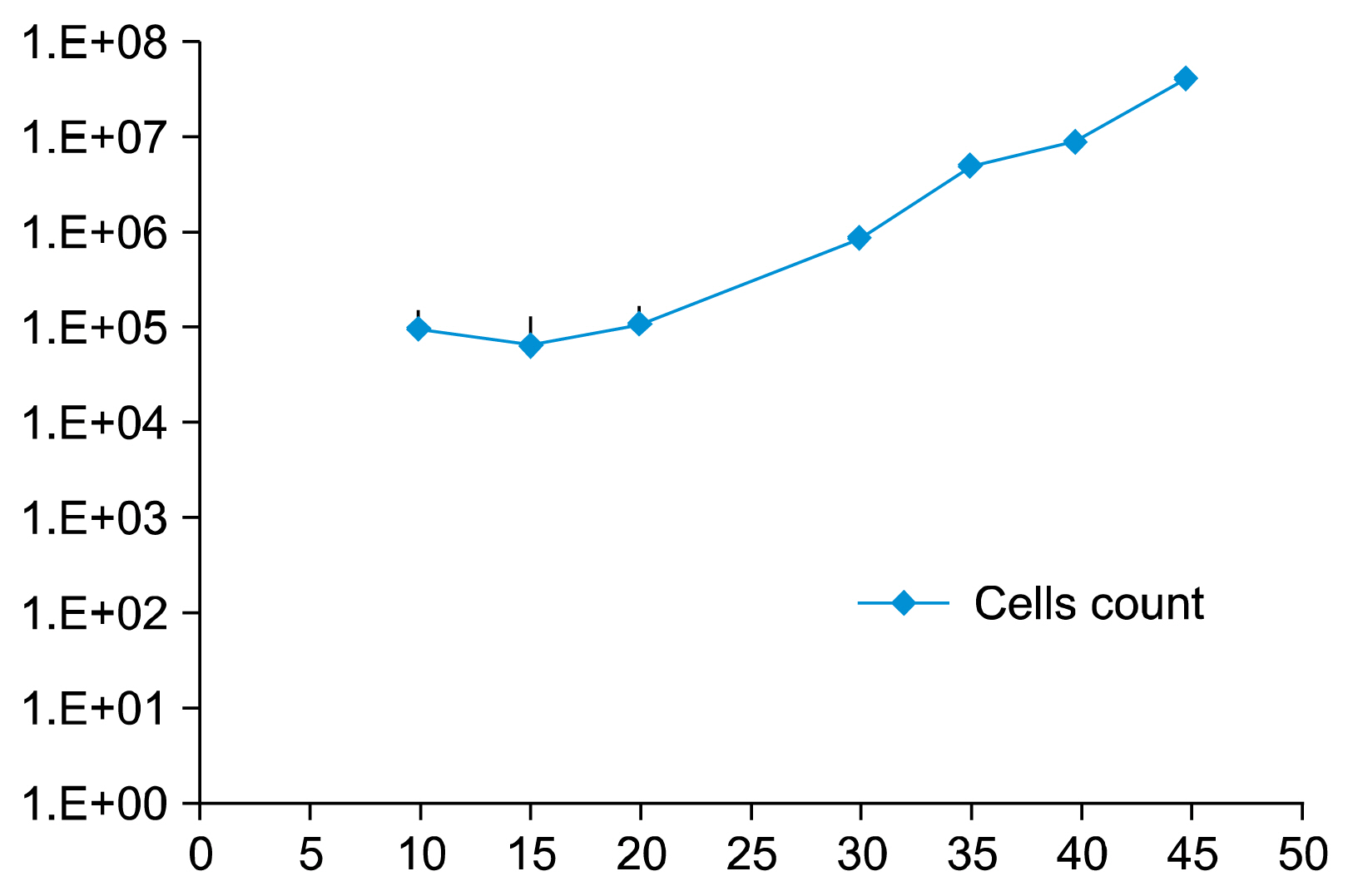
Cells transfected with SPI-1 continued to proliferate in a liquid culture media. Fluorescence-activated cell sorting (FACS) analysis on culture day 45 revealed a single population of CD71+CD117+ proerythroblast cells. The results of this study suggest that genetically modified erythroblasts could be immortalized in vitro by way of a system modeling murine erythroleukemia.
CONCLUSION
Genetic modification can increase erythroblast expansion capacity and potentially enable mass production of red blood cells.
MeSH Terms
Figure
Reference
-
References
1. The 2007 National Blood Collection and Utilization Survey Report. Washington, DC: DHHS;2008.2. Benjamin RJ, Whitaker BI. Boom or bust? Estimating blood demand and supply as the baby boomers age. Transfusion. 2011; 51:670–673. DOI: 10.1111/j.1537-2995.2011.03071.x. PMID: 21496037.
Article3. Surgenor DM, Wallace EL, Hao SHS, Chapman RH. Collection and transfusion of blood in the United States, 1982–1988. N Engl J Med. 1990; 322:1646–1651. DOI: 10.1056/NEJM199006073222306. PMID: 2342524.
Article4. Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993; 33:139–144. DOI: 10.1046/j.1537-2995.1993.33293158046.x. PMID: 8430453.
Article5. Wallace EL, Churchill WH, Surgenor DM, An J, Cho G, McGurk S, Murphy L. Collection and transfusion of blood and blood components in the United States, 1992. Transfusion. 1995; 35:802–812. DOI: 10.1046/j.1537-2995.1995.351096026360.x. PMID: 7570909.
Article6. Sullivan MT, McCullough J, Schreiber GB, Wallace EL. Blood collection and transfusion in the United States in 1997. Transfusion. 2002; 42:1253–1260. DOI: 10.1046/j.1537-2995.2002.00203.x. PMID: 12423507.
Article7. Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005; 45:141–148. DOI: 10.1111/j.1537-2995.2004.03288.x. PMID: 15660821.
Article8. Blood Facts and Statistics. American Red Cross [Internet]. Available from: www.redcrossblood.org/learn-about-blood/blood-facts-and-statistics.9. Geiler C, Andrade I, Greenwald D. Exogenous c-Myc blocks differentiation and improves expansion of human erythroblasts in vitro. Int J Stem Cells. 2014; 7:153–157. DOI: 10.15283/ijsc.2014.7.2.153. PMID: 25473453. PMCID: 4249898.
Article10. Migliaccio G, Di Pietro R, di Giacomo V, Di Baldassarre A, Migliaccio AR, Maccioni L, Galanello R, Papayannopoulou T. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol Dis. 2002; 28:169–180. DOI: 10.1006/bcmd.2002.0502. PMID: 12064913.
Article11. Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, Le Pennec PY, François S, Trugnan G, Peyrard T, Marie T, Jolly S, Hebert N, Mazurier C, Mario N, Harmand L, Lapillonne H, Devaux JY, Douay L. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011; 118:5071–5079. DOI: 10.1182/blood-2011-06-362038. PMID: 21885599. PMCID: 3217398.
Article12. Leberbauer C, Boulmé F, Unfried G, Huber J, Beug H, Müllner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005; 105:85–94. DOI: 10.1182/blood-2004-03-1002.
Article13. da Silva CL, Gonçalves R, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2005; 33:828–835. DOI: 10.1016/j.exphem.2005.03.017. PMID: 15963859.
Article14. Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006; 24:1255–1256. DOI: 10.1038/nbt1245. PMID: 16980975.
Article15. Fujimi A, Matsunaga T, Kobune M, Kawano Y, Nagaya T, Tanaka I, Iyama S, Hayashi T, Sato T, Miyanishi K, Sagawa T, Sato Y, Takimoto R, Takayama T, Kato J, Gasa S, Sakai H, Tsuchida E, Ikebuchi K, Hamada H, Niitsu Y. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008; 87:339–350. DOI: 10.1007/s12185-008-0062-y. PMID: 18369691.
Article16. Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011; 117:6083–6090. DOI: 10.1182/blood-2011-01-283606. PMID: 21436068. PMCID: 3122936.
Article17. Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001; 97:3075–3085. DOI: 10.1182/blood.V97.10.3075. PMID: 11342433.
Article18. Zamai L, Secchiero P, Pierpaoli S, Bassini A, Papa S, Alnemri ES, Guidotti L, Vitale M, Zauli G. TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesis. Blood. 2000; 95:3716–3724. PMID: 10845902.19. Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005; 15:146–155. DOI: 10.1016/j.tcb.2005.01.007. PMID: 15752978.
Article20. Lodish H, Flygare J, Chou S. From stem cell to erythroblast: regulation of red cell production at multiple levels by multiple hormones. IUBMB Life. 2010; 62:492–496. DOI: 10.1002/iub.322. PMID: 20306512. PMCID: 2893266.
Article21. Stephenson JR, Axelrad AA, McLeod DL, Shreeve MM. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971; 68:1542–1546. DOI: 10.1073/pnas.68.7.1542. PMID: 4104431. PMCID: 389236.
Article22. Nathan DG, Chess L, Hillman DG, Clarke B, Breard J, Merler E, Housman DE. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978; 147:324–339. DOI: 10.1084/jem.147.2.324. PMID: 304881. PMCID: 2184491.
Article23. Burda P, Laslo P, Stopka T. The role of PU.1 and GATA-1 transcription factors during normal and leukemogenic hematopoiesis. Leukemia. 2010; 24:1249–1257. DOI: 10.1038/leu.2010.104. PMID: 20520638.
Article24. Cook WD, McCaw BJ, Herring C, John DL, Foote SJ, Nutt SL, Adams JM. PU.1 is a suppressor of myeloid leukemia, inactivated in mice by gene deletion and mutation of its DNA binding domain. Blood. 2004; 104:3437–3444. DOI: 10.1182/blood-2004-06-2234. PMID: 15304397.
Article25. Barnache S, Wendling F, Lacombe C, Denis N, Titeux M, Vainchenker W, Moreau-Gachelin F. Spi-1 transgenic mice develop a clonal erythroleukemia which does not depend on p53 mutation. Oncogene. 1998; 16:2989–2995. DOI: 10.1038/sj.onc.1202095. PMID: 9662331.
Article26. Moreau-Gachelin F, Wendling F, Molina T, Denis N, Titeux M, Grimber G, Briand P, Vainchenker W, Tavitian A. Spi-1/PU.1 transgenic mice develop multistep erythroleukemias. Mol Cell Biol. 1996; 16:2453–2463. DOI: 10.1128/MCB.16.5.2453. PMID: 8628313. PMCID: 231234.
Article27. Ruscetti SK. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int J Biochem Cell Biol. 1999; 31:1089–1109. DOI: 10.1016/S1357-2725(99)00074-6. PMID: 10582341.
Article28. Chew SK, Rad R, Futreal PA, Bradley A, Liu P. Genetic screens using the piggyBac transposon. Methods. 2011; 53:366–371. DOI: 10.1016/j.ymeth.2010.12.022.
Article29. Wang W, Lin C, Lu D, Ning Z, Cox T, Melvin D, Wang X, Bradley A, Liu P. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2008; 105:9290–9295. DOI: 10.1073/pnas.0801017105. PMID: 18579772. PMCID: 2440425.30. Brendt P, Rehfeld I, Kamphausen A, Kreissig C, Peters J. Lipopolysaccharide interference in erythropoiesis in mice. Anaesthesia. 2012; 67:493–500. DOI: 10.1111/j.1365-2044.2011.07001.x. PMID: 22352462.
Article31. Fajtova M, Kovarikova A, Svec P, Kankuri E, Sedlak J. Immunophenotypic profile of nucleated erythroid progenitors during maturation in regenerating bone marrow. Leuk Lymphoma. 2013; 54:2523–2530. DOI: 10.3109/10428194.2013.781167. PMID: 23452116.
Article32. Erber WN, McLachlan J, Cordell JL, Turley H, Reid M, Mason DY. A new monoclonal antibody (JC159) that detects glycophorin A for the diagnosis of erythroleukaemia. Hematol Rev. 1991; 5:113–120.33. Gupta P, Gurudutta GU, Saluja D, Tripathi RP. PU.1 and partners: regulation of haematopoietic stem cell fate in normal and malignant haematopoiesis. J Cell Mol Med. 2009; 13:4349–4363. DOI: 10.1111/j.1582-4934.2009.00757.x. PMID: 19382896. PMCID: 4515051.
Article34. Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, White H, Zoi C, Loukopoulos D, Terpos E, Vervessou EC, Schultheis B, Emig M, Ernst T, Lengfelder E, Hehlmann R, Hochhaus A, Oscier D, Silver RT, Reiter A, Cross NC. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005; 106:2162–2168. DOI: 10.1182/blood-2005-03-1320. PMID: 15920007.
Article35. Irino T, Uemura M, Yamane H, Umemura S, Utsumi T, Kakazu N, Shirakawa T, Ito M, Suzuki T, Kinoshita K. JAK2 V617F-dependent upregulation of PU.1 expression in the peripheral blood of myeloproliferative neoplasm patients. PLoS One. 2011; 6:e22148. DOI: 10.1371/journal.pone.0022148. PMID: 21789226. PMCID: 3138766.
Article36. Fisher RC, Slayton WB, Chien C, Guthrie SM, Bray C, Scott EW. PU.1 supports proliferation of immature erythroid progenitors. Leuk Res. 2004; 28:83–89. DOI: 10.1016/S0145-2126(03)00178-4.
Article37. Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992; 89:5547–5551. DOI: 10.1073/pnas.89.12.5547. PMID: 1319065. PMCID: 49329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cryopreservation of mouse resources
- Genetically Engineered Mouse Models for Drug Development and Preclinical Trials
- Exogenous c-Myc Blocks Differentiation and Improves Expansion of Human Erythroblasts In vitro
- Reproductive performance of genetically engineered mice housed in different housing systems
- Hemoglobin Variability Associated with Erythropoiesis Stimulating Agents