Yonsei Med J.
2015 Sep;56(5):1307-1315. 10.3349/ymj.2015.56.5.1307.
TWIK-Related Spinal Cord K+ Channel Expression Is Increased in the Spinal Dorsal Horn after Spinal Nerve Ligation
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, Chungnam National University Hospital, Daejeon, Korea. annn8432@gmail.com, whlee@cnu.ac.kr
- 2Department of Anesthesia and Pain Medicine, Chungnam National University Medical School, Daejeon, Korea.
- 3Department of Anatomy, Chungnam National University Medical School, Daejeon, Korea.
- KMID: 2163622
- DOI: http://doi.org/10.3349/ymj.2015.56.5.1307
Abstract
- PURPOSE
The TWIK-related spinal cord K+ channel (TRESK) has recently been discovered and plays an important role in nociceptor excitability in the pain pathway. Because there have been no reports on the TRESK expression or its function in the dorsal horn of the spinal cord in neuropathic pain, we analyzed TRESK expression in the spinal dorsal horn in a spinal nerve ligation (SNL) model.
MATERIALS AND METHODS
We established a SNL mouse model by using the L5-6 spinal nerves ligation. We used real-time polymerase chain reaction and immunohistochemistry to investigate TRESK expression in the dorsal horn and L5 dorsal rot ganglion (DRG).
RESULTS
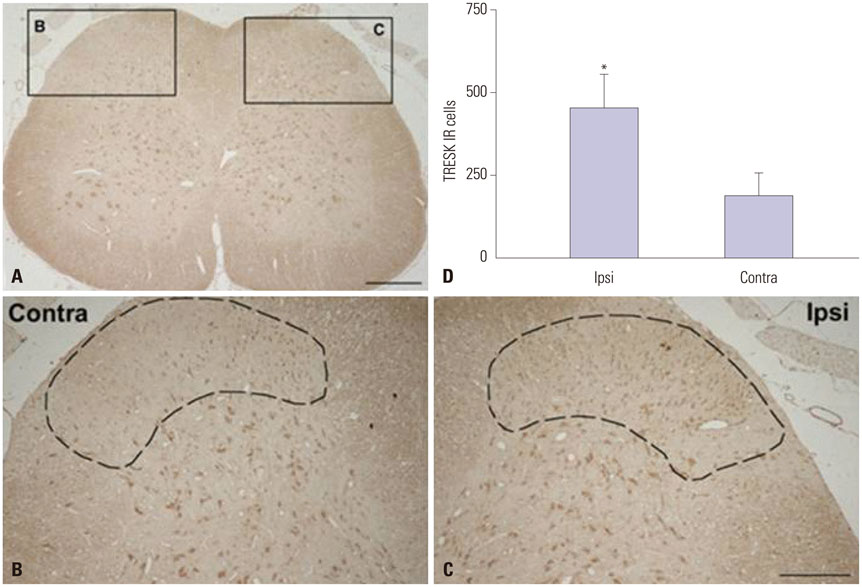
The SNL group showed significantly higher expression of TRESK in the ipsilateral dorsal horn under pain, but low expression in L5 DRG. Double immunofluorescence staining revealed that immunoreactivity of TRESK was mostly restricted in neuronal cells, and that synapse markers GAD67 and VGlut2 appeared to be associated with TRESK expression. We were unable to find a significant association between TRESK and calcineurin by double immunofluorescence.
CONCLUSION
TRESK in spinal cord neurons may contribute to the development of neuropathic pain following injury.
Keyword
MeSH Terms
-
Animals
Disease Models, Animal
Hyperalgesia
Ligation
Male
Neuralgia/*metabolism/physiopathology
Neurons/metabolism
Nociceptors
Pain/metabolism/*physiopathology
Potassium Channels/*metabolism
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
Spinal Cord Dorsal Horn/*metabolism
Spinal Nerves/*injuries
Potassium Channels
Figure
Reference
-
1. Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005; 11:2717–2736.
Article2. Ocaña M, Cendán CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. 2004; 500:203–219.
Article3. Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010; 90:559–605.
Article4. Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain. 2011; 7:30.
Article5. Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract. 2002; 2:87–97.
Article6. Song XJ, Vizcarra C, Xu DS, Rupert RL, Wong ZN. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol. 2003; 89:2185–2193.
Article7. Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009; 89:707–758.
Article8. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001; 429:23–37.
Article9. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363.
Article10. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.
Article11. Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001; 85:630–643.
Article12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article13. Czirják G, Enyedi P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J Biol Chem. 2006; 281:14677–14682.
Article14. Abdulla FA, Smith PA. Changes in Na(+) channel currents of rat dorsal root ganglion neurons following axotomy and axotomy-induced autotomy. J Neurophysiol. 2002; 88:2518–2529.
Article15. Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001; 85:644–658.
Article16. Delmas P. SnapShot: ion channels and pain. Cell. 2008; 134:366.
Article17. Liu C, Au JD, Zou HL, Cotten JF, Yost CS. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesth Analg. 2004; 99:1715–1722.
Article18. Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, et al. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003; 278:27406–27412.
Article19. Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006; 291:C138–C146.20. Smith HS. Calcineurin as a nociceptor modulator. Pain Physician. 2009; 12:E309–E318.
Article21. Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci. 2012; 49:375–386.
Article22. Wu X, Liu Y, Chen X, Sun Q, Tang R, Wang W, et al. Involvement of TREK-1 activity in astrocyte function and neuroprotection under simulated ischemia conditions. J Mol Neurosci. 2013; 49:499–506.
Article23. Zhou J, Yang CX, Zhong JY, Wang HB. Intrathecal TRESK gene recombinant adenovirus attenuates spared nerve injury-induced neuropathic pain in rats. Neuroreport. 2013; 24:131–136.
Article24. Berliocchi L, Russo R, Tassorelli C, Morrone LA, Bagetta G, Corasaniti MT. Death in pain: peripheral nerve injury and spinal neurodegenerative mechanisms. Curr Opin Pharmacol. 2012; 12:49–54.
Article25. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965; 150:971–979.
Article26. Fuccio C, Luongo C, Capodanno P, Giordano C, Scafuro MA, Siniscalco D, et al. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur J Pharmacol. 2009; 603:42–49.
Article27. Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005; 25:7317–7323.
Article28. Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990; 42:205–213.
Article29. Whiteside GT, Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J Neurosci Res. 2001; 64:168–173.
Article30. Leong ML, Gu M, Speltz-Paiz R, Stahura EI, Mottey N, Steer CJ, et al. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci. 2011; 31:17028–17039.
Article31. Czirják G, Tóth ZE, Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem. 2004; 279:18550–18558.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on the nNOS Expression in the Rat Spinal Cord of the Spinal Nerve Ligation Model with Neuropathic Pain and the Dorsal Rhizotomy
- G protein-coupled receptor, family C, group 5 (GPRC5B) downregulation in spinal cord neurons is involved in neuropathic pain
- Distribution of Tyrosine Hydroxylse Immunoreactive Structure in the Spinal Cord and Dorsal Root Ganglion of the Rat
- Change in the Expression of p75 Neurotrophin Receptor and TRPV1 in the Spinal Cord and Dorsal Root Ganglion after an Injury to the Spinal Nerves in Rats
- Antinociceptive Effects of Amiloride and Benzamil in Neuropathic Pain Model Rats