Yonsei Med J.
2015 Sep;56(5):1296-1306. 10.3349/ymj.2015.56.5.1296.
Serum Dickkopf-1 as a Biomarker for the Diagnosis of Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. gihankhys@yuhs.ac
- 2Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea. jhpark5277@yuhs.ac
- 4Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Nuclear Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 7Liver Cirrhosis Clinical Research Center, Seoul, Korea.
- 8Brain Korea 21 PLUS Project for Medical Science, Seoul, Korea.
- KMID: 2163621
- DOI: http://doi.org/10.3349/ymj.2015.56.5.1296
Abstract
- PURPOSE
Dickkopf-1 (DKK-1) is a Wnt/beta-catenin signaling pathway inhibitor. We investigated whether DKK-1 is related to progression in hepatocellular carcinoma (HCC) cells and HCC patients.
MATERIALS AND METHODS
In vitro reverse-transcription polymerase chain reaction (RT-PCR), wound healing assays, invasion assays, and ELISAs of patient serum samples were employed. The diagnostic accuracy of the serum DKK-1 ELISA was assessed using receiver operating characteristic (ROC) curves and area under ROC (AUC) analyses.
RESULTS
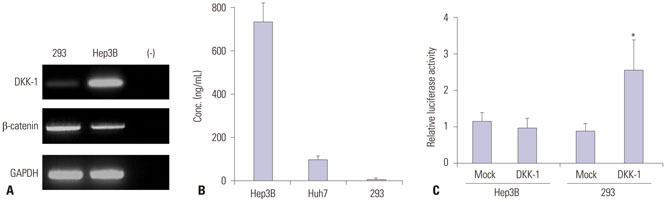
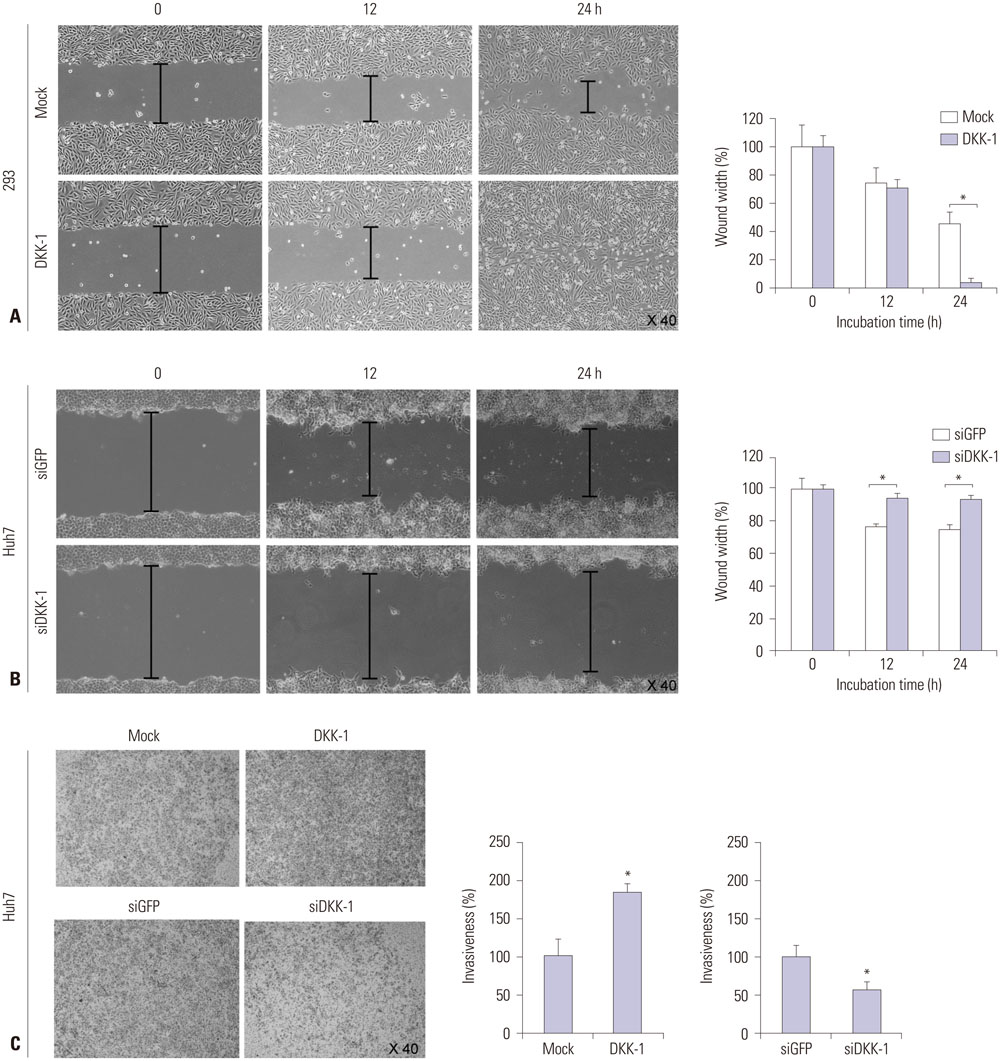
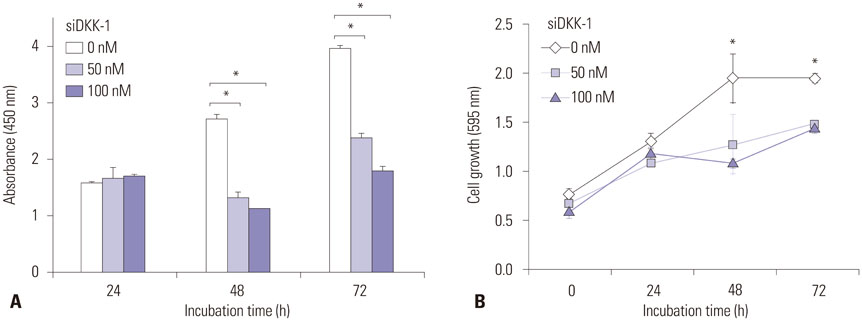
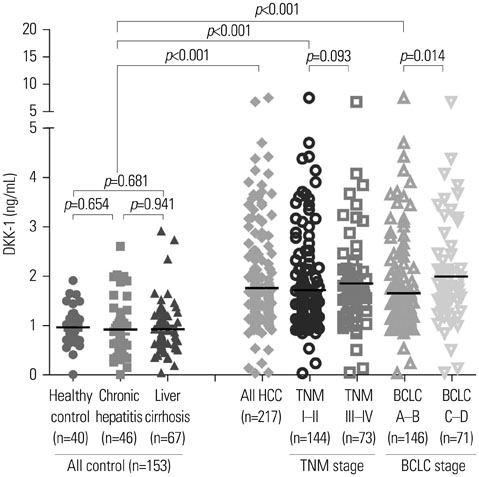
RT-PCR showed high DKK-1 expression in Hep3B and low in 293 cells. Similarly, the secreted DKK-1 concentration in the culture media was high in Hep3B and low in 293 cells. Wound healing and invasion assays using 293, Huh7, and Hep3B cells showed that DKK-1 overexpression promoted cell migration and invasion, whereas DKK-1 knock-down inhibited them. When serum DKK-1 levels were assessed in 370 participants (217 with HCC and 153 without), it was significantly higher in HCC patients than in control groups (median 1.48 ng/mL vs. 0.90 ng/mL, p<0.001). The optimum DKK-1 cutoff level was 1.01 ng/mL (AUC=0.829; sensitivity 90.7%; specificity 62.0%). Although DKK-1 had a higher AUC than alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) (AUC=0.829 vs. 0.794 and 0.815, respectively), they were statistically similar (all p>0.05). When three biomarkers were combined (DKK-1 plus AFP plus DCP), they showed significantly higher AUC (AUC=0.952) than single marker, DKK-1 plus AFP, or DKK-1 plus DCP (all p<0.001).
CONCLUSION
DKK-1 might be a key regulator in HCC progression and a potential therapeutic target in HCC. Serum DKK-1 could complement the diagnostic accuracy of AFP and DCP.
Keyword
MeSH Terms
-
Area Under Curve
Biomarkers/blood/metabolism
Biomarkers, Tumor/blood
Carcinoma, Hepatocellular/blood/*diagnosis
Enzyme-Linked Immunosorbent Assay
Female
Humans
Intercellular Signaling Peptides and Proteins/*blood/*metabolism
Liver Neoplasms/blood/*diagnosis
Male
Middle Aged
Protein Precursors/blood/metabolism
Prothrombin/metabolism
ROC Curve
Reverse Transcriptase Polymerase Chain Reaction/*methods
Sensitivity and Specificity
alpha-Fetoproteins/analysis/metabolism
Biomarkers
Biomarkers, Tumor
Intercellular Signaling Peptides and Proteins
Protein Precursors
Prothrombin
alpha-Fetoproteins
Figure
Cited by 1 articles
-
Soluble Axl Is a Novel Diagnostic Biomarker of Hepatocellular Carcinoma in Chinese Patients with Chronic Hepatitis B Virus Infection
Xiaoting Song, Ailu Wu, Zhixiao Ding, Shixiong Liang, Chunyan Zhang
Cancer Res Treat. 2020;52(3):789-797. doi: 10.4143/crt.2019.749.
Reference
-
1. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001; 35:421–430.
Article2. Kim SU, Kim do Y, Park JY, Ahn SH, Nah HJ, Chon CY, et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J Cancer Res Clin Oncol. 2008; 134:1377–1384.
Article3. Wands JR, Blum HE. Primary hepatocellular carcinoma. N Engl J Med. 1991; 325:729–731.
Article4. Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009; 27:80–92.5. Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006; 25:3771–3777.
Article6. Zucman-Rossi J, Laurent-Puig P. Genetic diversity of hepatocellular carcinomas and its potential impact on targeted therapies. Pharmacogenomics. 2007; 8:997–1003.
Article7. Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007; 45:1298–1305.8. Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996; 382:595–601.
Article9. Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997; 88:757–766.
Article10. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998; 391:357–362.
Article11. Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999; 274:19465–19472.
Article12. Zorn AM. Wnt signalling: antagonistic Dickkopfs. Curr Biol. 2001; 11:R592–R595.
Article13. Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001; 11:951–961.
Article14. Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002; 417:664–667.
Article15. González-Sancho JM, Aguilera O, García JM, Pendás-Franco N, Peña C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005; 24:1098–1103.
Article16. Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002; 21:878–889.
Article17. Wirths O, Waha A, Weggen S, Schirmacher P, Kühne T, Goodyer CG, et al. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms' tumors. Lab Invest. 2003; 83:429–434.
Article18. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003; 349:2483–2494.
Article19. Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, et al. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007; 96:646–653.
Article20. Patil MA, Chua MS, Pan KH, Lin R, Lih CJ, Cheung ST, et al. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005; 24:3737–3747.
Article21. Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008; 68:1451–1461.
Article22. Lee JD, Yun M, Lee JM, Choi Y, Choi YH, Kim JS, et al. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur J Nucl Med Mol Imaging. 2004; 31:1621–1630.
Article23. Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009; 50:948–957.
Article24. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
Article25. Chun YH, Kim SU, Park JY, Kim do Y, Han KH, Chon CY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011; 47:2568–2575.
Article26. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010; 30:61–74.
Article27. Kim do Y, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, et al. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci. 2009; 54:1758–1763.
Article28. Kim SU, Han KH, Nam CM, Park JY, Kim do Y, Chon CY, et al. Natural history of hepatitis B virus-related cirrhotic patients hospitalized to control ascites. J Gastroenterol Hepatol. 2008; 23:1722–1727.
Article29. Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009; 137:850–855.30. Fuster J, García-Valdecasas JC, Grande L, Tabet J, Bruix J, Anglada T, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg. 1996; 223:297–302.31. Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995; 19:1409–1417.32. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245–1255.
Article33. Wu W, Yao DF, Yuan YM, Fan JW, Lu XF, Li XH, et al. Combined serum hepatoma-specific alpha-fetoprotein and circulating alpha-fetoprotein-mRNA in diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006; 5:538–544.34. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17:9–26.35. Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999; 238:301–313.
Article36. Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010; 70:5326–5336.
Article37. Shizhuo W, Tao J, Shulan Z, Bing Z. The expression and significance of Dickkopf-1 in epithelial ovarian carcinoma. Int J Biol Markers. 2009; 24:165–170.
Article38. Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009; 55:1656–1664.
Article39. Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012; 13:817–826.
Article40. Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010; 55:2744–2755.
Article41. Kuang HB, Miao CL, Guo WX, Peng S, Cao YJ, Duan EK. Dickkopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Front Biosci (Landmark Ed). 2009; 14:2212–2220.
Article42. Kwack MH, Hwang SY, Jang IS, Im SU, Kim JO, Kim MK, et al. Analysis of cellular changes resulting from forced expression of Dickkopf-1 in hepatocellular carcinoma cells. Cancer Res Treat. 2007; 39:30–36.
Article43. Fujiyama S, Tanaka M, Maeda S, Ashihara H, Hirata R, Tomita K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology. 2002; 62:Suppl 1. 57–63.
Article44. Tung EK, Mak CK, Fatima S, Lo RC, Zhao H, Zhang C, et al. Clinicopathological and prognostic significance of serum and tissue Dickkopf-1 levels in human hepatocellular carcinoma. Liver Int. 2011; 31:1494–1504.
Article45. Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008; 68:1396–1404.
Article46. Tao YM, Liu Z, Liu HL. Dickkopf-1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig Liver Dis. 2013; 45:251–257.
Article47. Glaw JT, Skalak TC, Peirce SM. Inhibition of canonical Wnt signaling increases microvascular hemorrhaging and venular remodeling in adult rats. Microcirculation. 2010; 17:348–357.
Article48. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Cellular Changes Resulting from Forced Expression of Dickkopf-1 in Hepatocellular Carcinoma Cells
- Diagnosis of hepatocellular carcinoma: high risk population and serum alpha-alpha-fetoprotein determination
- Noninvasive diagnostic criteria for hepatocellular carcinoma
- Imaging Diagnosis of Hepatocellular Carcinoma
- Kupffer Cells in Hepatocellular Carcinoma