Ann Surg Treat Res.
2016 May;90(5):279-286. 10.4174/astr.2016.90.5.279.
Intraoperative indocyanine green angiography for the objective measurement of blood flow
- Affiliations
-
- 1Department of Surgery, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea. jhjoh@khu.ac.kr
- 2Department of Surgery, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 2163068
- DOI: http://doi.org/10.4174/astr.2016.90.5.279
Abstract
- PURPOSE
Intraoperative assessment of tissue perfusion is important to predict wound healing or improvement of symptoms in patients with peripheral arterial disease (PAD) or vascular trauma. There is no widely accepted standard for intraoperative measurement of tissue perfusion. Here, we report the use of indocyanine green (ICG)-based angiography to determine the blood flow in patients with PAD and vascular trauma.
METHODS
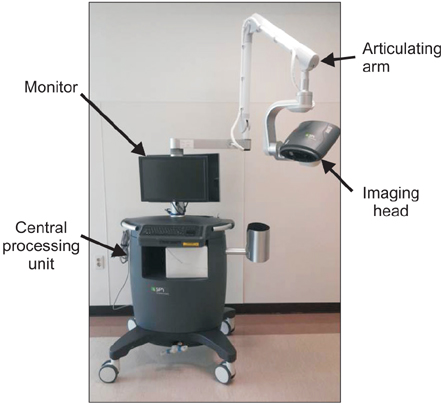
The SPY fluorescent imaging system was utilized. A dose of 3-5 mL of ICG (2.5 mg/mL) was injected intravenously followed by a 10 mL normal saline flush. The SPY imaging system was used to quantitatively assess perfusion. During the study period, the SPY imaging system was applied in 4 patients with PAD and one patient with vascular trauma.
RESULTS
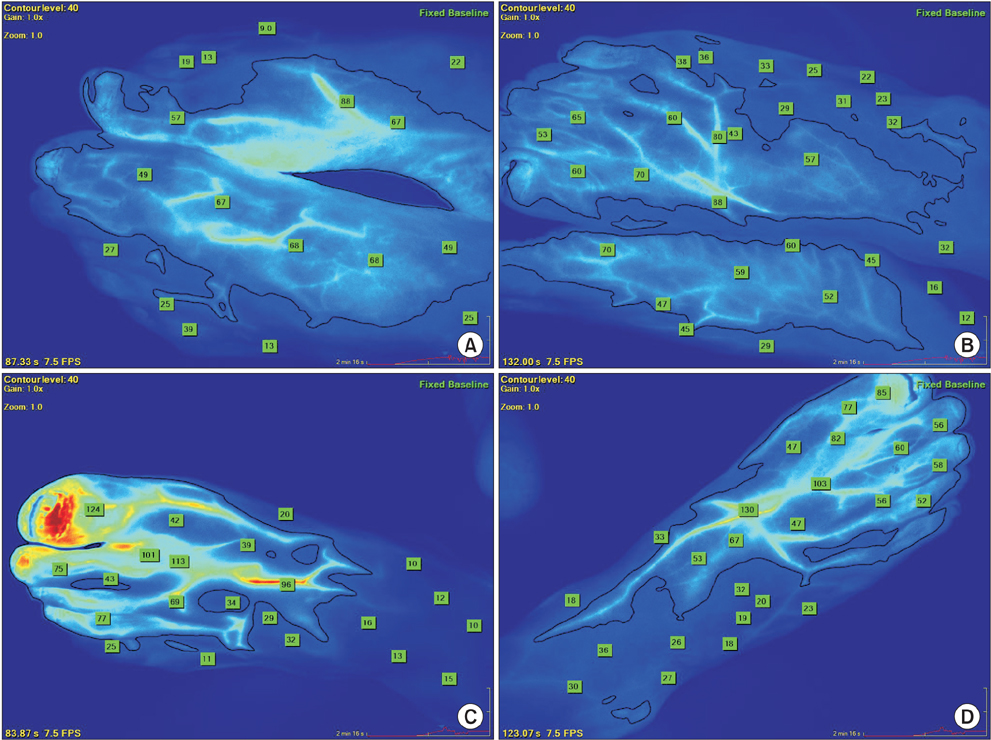
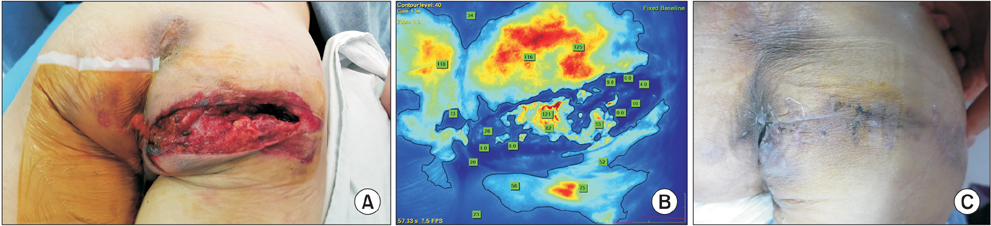
In 3 patients with PAD associated with an ischemic wound, complete wound healing was achieved with the indication of viable tissue by the SPY system. In one patient with severe claudication in both lower extremities, the ICG angiography was used to determine the increased blood flow after revascularization. In the case of vascular trauma, this imaging system enabled the delineation of viability of the injured tissue.
CONCLUSION
ICG angiography can determine the surface tissue viability in PAD patients. In cases of severe vascular trauma,the SPY system can be used to determine tissue perfusion. Further study is warranted to define the definite utility of this technology to assess perfusion, response to revascularization, and potentially, to predict the likelihood of wound healing.
MeSH Terms
Figure
Reference
-
1. Ahn S, Park YJ, Min SI, Kim SY, Ha J, Kim SJ, et al. High prevalence of peripheral arterial disease in Korean patients with coronary or cerebrovascular disease. J Korean Med Sci. 2012; 27:625–629.2. Lee JY, Lee SW, Lee WS, Han S, Park YK, Kwon CH, et al. Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv. 2013; 6:1303–1313.3. Park IH, Lee SC, Park IS, Nam CH, Ahn HS, Park HY, et al. Asymptomatic peripheral vascular disease in total knee arthroplasty: preoperative prevalence and risk factors. J Orthop Traumatol. 2015; 16:23–26.4. Jallali N, Ridha H, Butler PE. Postoperative monitoring of free flaps in UK plastic surgery units. Microsurgery. 2005; 25:469–472.5. Mothes H, Dönicke T, Friedel R, Simon M, Bach O, Markgraf E. Indocyanine-green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma. 2004; 57:1018–1024.6. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008; 31:1679–1685.7. Gurtner GC, Jones GE, Neligan PC, Newman MI, Phillips BT, Sacks JM, et al. Intraoperative laser angiography using the SPY system: review of the literature and recommendations for use. Ann Surg Innov Res. 2013; 7:1.8. Patel KM, Bhanot P, Franklin B, Albino F, Nahabedian MY. Use of intraoperative indocyanin-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg. 2013; 47:476–480.9. Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960; 39:592–600.10. Pestana IA, Coan B, Erdmann D, Marcus J, Levin LS, Zenn MR. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg. 2009; 123:1239–1244.11. Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg. 2009; 25:21–26.12. Murray JD, Jones GE, Elwood ET, Whitty LA, Garcia C. Fluorescent intraoperative tissue angiography with indocyanine green: evaluation of nipple-areola vascularity during breast reduction surgery. Plast Reconstr Surg. 2010; 126:33e–34e.13. Munabi JD, Jones GE, Elwood ET, Whitty LA, Garcia C. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014; 67:449–455.14. Protyniak B, Dinallo AM, Boyan WP Jr, Dressner RM, Arvanitis ML. Intraoperative indocyanine green fluorescence angiography: an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg. 2015; 81:580–584.15. Ris F, Hompes R, Cunningham C, Lindsey I, Guy R, Jones O, et al. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc. 2014; 28:2221–2226.16. Waseda K, Ako J, Hasegawa T, Shimada Y, Ikeno F, Ishikawa T, et al. Intraoperative fluorescence imaging system for on-site assessment of off-pump coronary artery bypass graft. JACC Cardiovasc Imaging. 2009; 2:604–612.17. Yamamoto M, Orihashi K, Nishimori H, Wariishi S, Fukutomi T, Kondo N, et al. Indocyanine green angiography for intraoperative assessment in vascular surgery. Eur J Vasc Endovasc Surg. 2012; 43:204–208.18. Perry D, Bharara M, Armstrong DG, Mills J. Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol. 2012; 6:204–208.19. Braun JD, Trinidad-Hernandez M, Perry D, Armstrong DG, Mills JL Sr. Early quantitative evaluation of indocyanine green angiography in patients with critical limb ischemia. J Vasc Surg. 2013; 57:1213–1218.20. Connolly PH, Meltzer AJ, Spector JA, Schneider DB. Indocyanine green angiography aids in prediction of limb salvage in vascular trauma. Ann Vasc Surg. 2015; 29:1453e1–e4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indocyanine green angiography of retinal astrocytomas associated with tuberous sclerosis
- Utility of Indocyanine Green Fluorescence Imaging in Wound Assessment
- Quantitative Analysis of Intraoperative Indocyanine Green Video Angiography in Aneurysm Surgery
- Surgical considerations and techniques using intraoperative indocyanine green angiography for ethmoidal dural arteriovenous fistula
- Intraoperative assessment of parathyroid perfusion using indocyanine green angiography in robotic thyroidectomy