Diabetes Metab J.
2016 Apr;40(2):89-98. 10.4093/dmj.2016.40.2.89.
Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Biochemistry, Catholic Kwandong University College of Medicine, Gangneung, Korea.
- 3Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea. hailkim@kaist.edu
- KMID: 2162080
- DOI: http://doi.org/10.4093/dmj.2016.40.2.89
Abstract
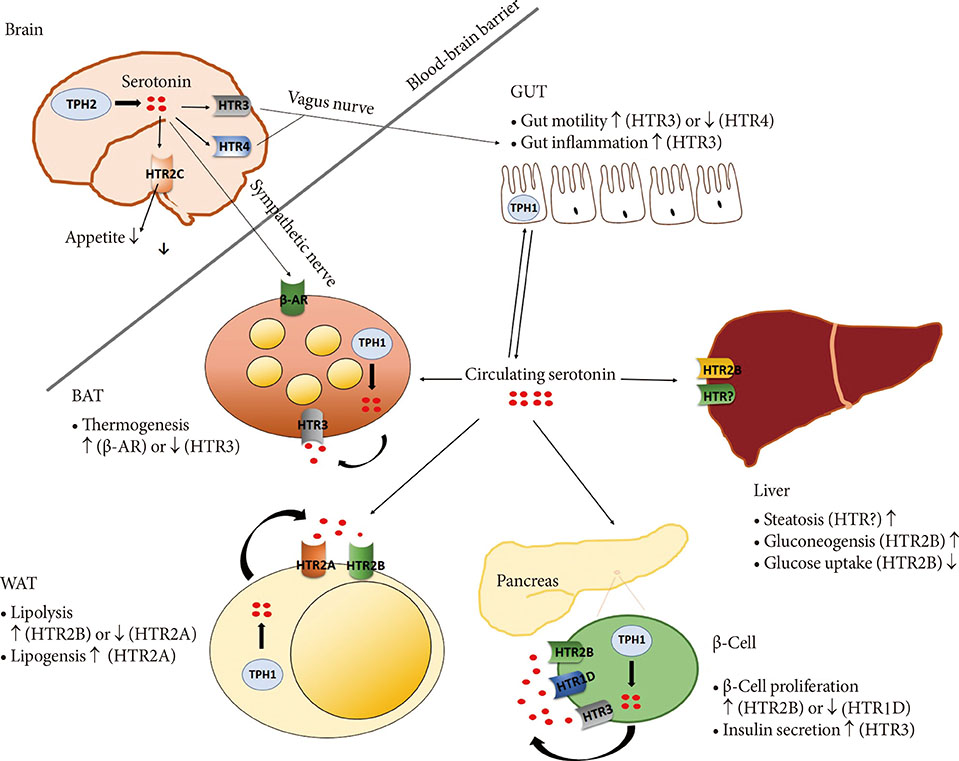
- Serotonin (5-hydroxytryptamine [5-HT]) is a monoamine that has various functions in both neuronal and non-neuronal systems. In the central nervous system, 5-HT regulates mood and feeding behaviors as a neurotransmitter. Thus, there have been many trials aimed at increasing the activity of 5-HT in the central nervous system, and some of the developed methods are already used in the clinical setting as anti-obesity drugs. Unfortunately, some drugs were withdrawn due to the development of unwanted peripheral side effects, such as valvular heart disease and pulmonary hypertension. Recent studies revealed that peripheral 5-HT plays an important role in metabolic regulation in peripheral tissues, where it suppresses adaptive thermogenesis in brown adipose tissue. Inhibition of 5-HT synthesis reduced the weight gain and improved the metabolic dysfunction in a diet-induced obesity mouse model. Genome-wide association studies also revealed genetic associations between the serotonergic system and obesity. Several genetic polymorphisms in tryptophan hydroxylase and 5-HT receptors were shown to have strong associations with obesity. These results support the clinical significance of the peripheral serotonergic system as a therapeutic target for obesity and diabetes.
Keyword
MeSH Terms
-
Adipose Tissue, Brown
Animals
Anti-Obesity Agents
Central Nervous System
Diabetes Mellitus*
Feeding Behavior
Genome-Wide Association Study
Heart Valve Diseases
Hypertension, Pulmonary
Mice
Neurons
Neurotransmitter Agents
Obesity*
Polymorphism, Genetic
Receptors, Serotonin
Serotonin*
Thermogenesis
Tryptophan Hydroxylase
Weight Gain
Anti-Obesity Agents
Neurotransmitter Agents
Receptors, Serotonin
Serotonin
Tryptophan Hydroxylase
Figure
Cited by 2 articles
-
The Effect of Antidepressants on Mesenchymal Stem Cell Differentiation
Jeffrey S. Kruk, Sandra Bermeo, Kristen K. Skarratt, Stephen J. Fuller, Gustavo Duque
J Bone Metab. 2018;25(1):43-51. doi: 10.11005/jbm.2018.25.1.43.Serotonergic Regulation of Hepatic Energy Metabolism
Jiwon Park, Wooju Jeong, Chahyeon Yun, Hail Kim, Chang-Myung Oh
Endocrinol Metab. 2021;36(6):1151-1160. doi: 10.3803/EnM.2021.1331.
Reference
-
1. Janeway TC, Richardson HB, Park EA. Experiments on the vasoconstrictor action of blood serum. Arch Intern Med. 1918; 21:565–603.2. Zucker MB. A study of the substances in blood serum and platelets which stimulate smooth muscle. Am J Physiol. 1944; 142:12–26.3. Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952; 169:800–801.4. Brodie BB, Shore PA. A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann N Y Acad Sci. 1957; 66:631–642.5. Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996; 16:2352–2364.6. Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005; 22:895–901.7. Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011; 31:8998–9009.8. Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012; 16:588–600.9. Ponicke K, Gergs U, Buchwalow IB, Hauptmann S, Neumann J. On the presence of serotonin in mammalian cardiomyocytes. Mol Cell Biochem. 2012; 365:301–312.10. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010; 16:804–808.11. Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, Park BY, Lee HW, Jeon YH, Song J, Shong M, Yadav VK, Karsenty G, Kajimura S, Lee IK, Park S, Kim H. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015; 6:6794.12. Peroutka SJ, Howell TA. The molecular evolution of G protein-coupled receptors: focus on 5-hydroxytryptamine receptors. Neuropharmacology. 1994; 33:319–324.13. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008; 31:187–199.14. Barnes NM, Neumaier JF. Neuronal 5-HT receptors and SERT. Tocris Biosci Sci Rev Ser. 2011; 34:1–16.15. Reeves DC, Lummis SC. The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review). Mol Membr Biol. 2002; 19:11–26.16. Lam DD, Heisler LK. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert Rev Mol Med. 2007; 9:1–24.17. Heal DJ, Aspley S, Prow MR, Jackson HC, Martin KF, Cheetham SC. Sibutramine: a novel anti-obesity drug. A review of the pharmacological evidence to differentiate it from d-amphetamine and d-fenfluramine. Int J Obes Relat Metab Disord. 1998; 22:Suppl 1. S18–S28.18. Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav. 1997; 58:767–773.19. Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine. Science. 1976; 192:382–385.20. Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors: hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl). 1988; 96:93–100.21. Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995; 374:542–546.22. Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998; 4:1152–1156.23. Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002; 297:609–611.24. Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010; 363:245–256.25. CinicalTrials.gov: A service of the U.S. National Insititutes of Health. cited 2016 Jan 11. Available from: https://www.clinicaltrials.gov.26. U.S. Food and Drug Administration. FDA approves Belviq to treat some overweight or obese adults. Home Healthc Nurse. 2012; 30:443–444.27. Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS One. 2008; 3:e3301.28. Gutknecht L, Araragi N, Merker S, Waider J, Sommerlandt FM, Mlinar B, Baccini G, Mayer U, Proft F, Hamon M, Schmitt AG, Corradetti R, Lanfumey L, Lesch KP. Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS One. 2012; 7:e43157.29. Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 2001; 74:507–516.30. Le Feuvre RA, Aisenthal L, Rothwell NJ. Involvement of corticotrophin releasing factor (CRF) in the thermogenic and anorexic actions of serotonin (5-HT) and related compounds. Brain Res. 1991; 555:245–250.31. Sakaguchi T, Bray GA. Effect of norepinephrine, serotonin and tryptophan on the firing rate of sympathetic nerves. Brain Res. 1989; 492:271–280.32. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009; 60:355–366.33. Amireault P, Sibon D, Cote F. Life without peripheral serotonin: insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks. ACS Chem Neurosci. 2013; 4:64–71.34. Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005; 39:5 Suppl 3. S184–S193.35. Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs. 1998; 55:173–189.36. Liang LX, Zhang Q, Qian W, Hou XH. Antinociceptive property of tegaserod in a rat model of chronic visceral hypersensitivity. Chin J Dig Dis. 2005; 6:21–25.37. Manocha M, Khan WI. Serotonin and GI disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012; 3:e13.38. O'Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006; 107:1010–1017.39. Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011; 11:29–33.40. Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007; 109:3139–3146.41. Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007; 31:808–816.42. Matondo RB, Punt C, Homberg J, Toussaint MJ, Kisjes R, Korporaal SJ, Akkerman JW, Cuppen E, de Bruin A. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G963–G968.43. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006; 312:104–107.44. Papadimas GK, Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Skaltsas SD, Hereti RI, Papadopoulou-Daifoti Z, Mykoniatis MG. Effect of serotonin receptor 2 blockage on liver regeneration after partial hepatectomy in the rat liver. Liver Int. 2006; 26:352–361.45. Tzirogiannis KN, Kourentzi KT, Zyga S, Papalimneou V, Tsironi M, Grypioti AD, Protopsaltis I, Panidis D, Panoutsopoulos GI. Effect of 5-HT7 receptor blockade on liver regeneration after 60-70% partial hepatectomy. BMC Gastroenterol. 2014; 14:201.46. Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blumer RM, Fullerton MD, Yabut JM, Kim JJ, Ghia JE, Hamza SM, Morrison KM, Schertzer JD, Dyck JR, Khan WI, Steinberg GR. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015; 21:166–172.47. Moore MC, DiCostanzo CA, Dardevet D, Lautz M, Farmer B, Neal DW, Cherrington AD. Portal infusion of a selective serotonin reuptake inhibitor enhances hepatic glucose disposal in conscious dogs. Am J Physiol Endocrinol Metab. 2004; 287:E1057–E1063.48. Haub S, Ritze Y, Ladel I, Saum K, Hubert A, Spruss A, Trautwein C, Bischoff SC. Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J Pharmacol Exp Ther. 2011; 339:790–798.49. Gershon MD, Ross LL. Location of sites of 5-hydroxytryptamine storage and metabolism by radioautography. J Physiol. 1966; 186:477–492.50. Ekholm R, Ericson LE, Lundquist I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971; 7:339–348.51. Kim K, Oh CM, Ohara-Imaizumi M, Park S, Namkung J, Yadav VK, Tamarina NA, Roe MW, Philipson LH, Karsenty G, Nagamatsu S, German MS, Kim H. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015; 156:444–452.52. Ohara-Imaizumi M, Kim H, Yoshida M, Fujiwara T, Aoyagi K, Toyofuku Y, Nakamichi Y, Nishiwaki C, Okamura T, Uchida T, Fujitani Y, Akagawa K, Kakei M, Watada H, German MS, Nagamatsu S. Serotonin regulates glucose-stimulated insulin secretion from pancreatic beta cells during pregnancy. Proc Natl Acad Sci U S A. 2013; 110:19420–19425.53. Ohta Y, Kosaka Y, Kishimoto N, Wang J, Smith SB, Honig G, Kim H, Gasa RM, Neubauer N, Liou A, Tecott LH, Deneris ES, German MS. Convergence of the insulin and serotonin programs in the pancreatic beta-cell. Diabetes. 2011; 60:3208–3216.54. Berger M, Scheel DW, Macias H, Miyatsuka T, Kim H, Hoang P, Ku GM, Honig G, Liou A, Tang Y, Regard JB, Sharifnia P, Yu L, Wang J, Coughlin SR, Conklin BR, Deneris ES, Tecott LH, German MS. Galphai/o-coupled receptor signaling restricts pancreatic beta-cell expansion. Proc Natl Acad Sci U S A. 2015; 112:2888–2893.55. Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008; 9:85–96.56. Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y, Takanabe-Mori R, Hasegawa K, Kita T, Kimura T. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol. 2010; 24:1978–1987.57. Gres S, Canteiro S, Mercader J, Carpene C. Oxidation of high doses of serotonin favors lipid accumulation in mouse and human fat cells. Mol Nutr Food Res. 2013; 57:1089–1099.58. Uchida-Kitajima S, Yamauchi T, Takashina Y, Okada-Iwabu M, Iwabu M, Ueki K, Kadowaki T. 5-Hydroxytryptamine 2A receptor signaling cascade modulates adiponectin and plasminogen activator inhibitor 1 expression in adipose tissue. FEBS Lett. 2008; 582:3037–3044.59. Nomura S, Shouzu A, Omoto S, Nishikawa M, Iwasaka T. 5-HT2A receptor antagonist increases circulating adiponectin in patients with type 2 diabetes. Blood Coagul Fibrinolysis. 2005; 16:423–428.60. Kwak SH, Park BL, Kim H, German MS, Go MJ, Jung HS, Koo BK, Cho YM, Choi SH, Cho YS, Shin HD, Jang HC, Park KS. Association of variations in TPH1 and HTR2B with gestational weight gain and measures of obesity. Obesity (Silver Spring). 2012; 20:233–238.61. Rosmond R, Bouchard C, Bjorntorp P. Increased abdominal obesity in subjects with a mutation in the 5-HT(2A) receptor gene promoter. Ann N Y Acad Sci. 2002; 967:571–575.62. Halder I, Muldoon MF, Ferrell RE, Manuck SB. Serotonin receptor 2A (HTR2A) gene polymorphisms are associated with blood pressure, central adiposity, and the metabolic syndrome. Metab Syndr Relat Disord. 2007; 5:323–330.63. Li P, Tiwari HK, Lin WY, Allison DB, Chung WK, Leibel RL, Yi N, Liu N. Genetic association analysis of 30 genes related to obesity in a European American population. Int J Obes (Lond). 2014; 38:724–729.64. Opgen-Rhein C, Brandl EJ, Muller DJ, Neuhaus AH, Tiwari AK, Sander T, Dettling M. Association of HTR2C, but not LEP or INSIG2, genes with antipsychotic-induced weight gain in a German sample. Pharmacogenomics. 2010; 11:773–780.65. Suviolahti E, Oksanen LJ, Ohman M, Cantor RM, Ridderstrale M, Tuomi T, Kaprio J, Rissanen A, Mustajoki P, Jousilahti P, Vartiainen E, Silander K, Kilpikari R, Salomaa V, Groop L, Kontula K, Peltonen L, Pajukanta P. The SLC6A14 gene shows evidence of association with obesity. J Clin Invest. 2003; 112:1762–1772.66. Corpeleijn E, Petersen L, Holst C, Saris WH, Astrup A, Langin D, MacDonald I, Martinez JA, Oppert JM, Polak J, Pedersen O, Froguel P, Arner P, Sorensen TI, Blaak EE. Obesity-related polymorphisms and their associations with the ability to regulate fat oxidation in obese Europeans: the NUGENOB study. Obesity (Silver Spring). 2010; 18:1369–1377.67. Chen C, Chen W, Chen C, Moyzis R, He Q, Lei X, Li J, Wang Y, Liu B, Xiu D, Zhu B, Dong Q. Genetic variations in the serotoninergic system contribute to body-mass index in Chinese adolescents. PLoS One. 2013; 8:e58717.68. Karsenty G, Sumara G, Sumara O. The Trustees of Columbia University in the City of New York, assignee. Methods of preventing and treating diabetes by inhibiting serotonin synthesis. United States patent. US 9,150,521. 2013. Aug. 8.69. Khan W, Steinberg G, Palanivel R. Mcmaster University, assignee. A method of treating obesity. Canada patent. CA 2900413A1. 2014. Aug. 21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Treatments on Obesity
- Obesity and Types Diabetes Mellitus
- The Effects of Abdominal Obesity on the Increased Prevalence Rate of Hypertension and Diabetes Mellitus in Benign Prostatic Hyperplasia Patients
- Management of Obesity in Patients with Diabetes Mellitus
- The Serotonin-6 Receptor as a Novel Therapeutic Target