J Vet Sci.
2015 Mar;16(1):67-74. 10.4142/jvs.2015.16.1.67.
Normal clinical electroretinography parameters for poodle, Labrador retriever, Thai ridgeback, and Thai Bangkaew
- Affiliations
-
- 1Center for Agricultural Biotechnology, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom 73140, Thailand.
- 2Center of Excellence on Agricultural Biotechnology (AG-BIO/PERDO-CHE), Bangkok 10900, Thailand.
- 3Department of Companion Animal Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom 73140, Thailand. fvetatn@ku.ac.th
- KMID: 2160822
- DOI: http://doi.org/10.4142/jvs.2015.16.1.67
Abstract
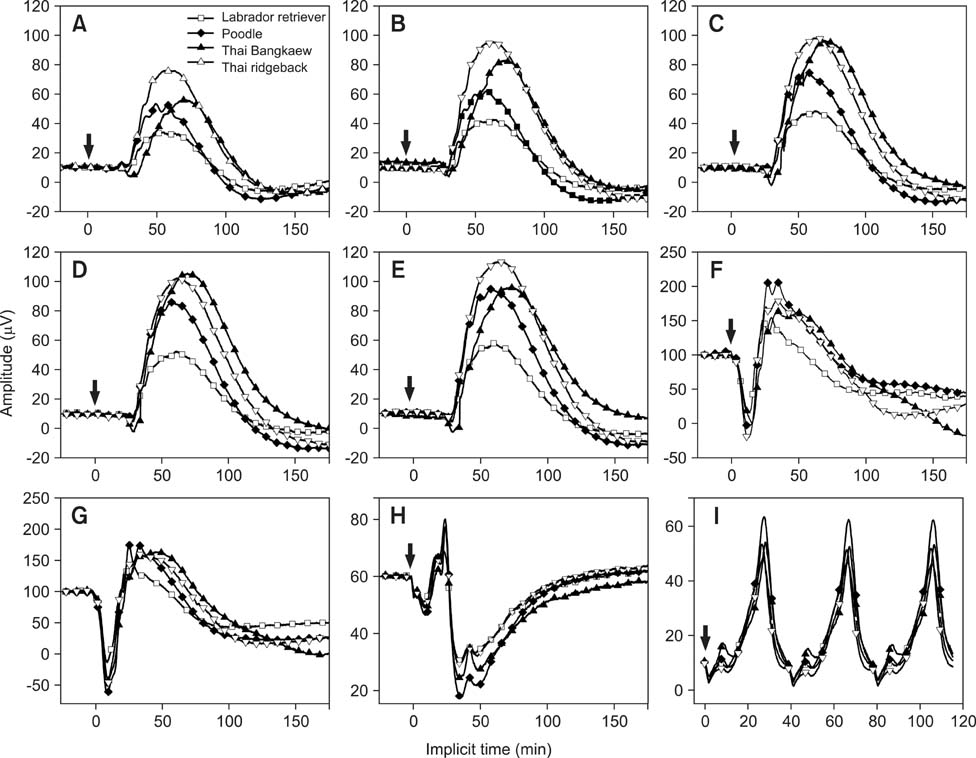
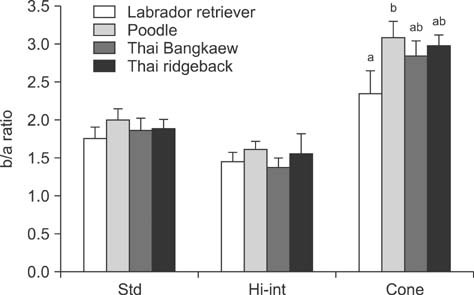
- The purpose of the present study was to establish normal electroretinogram (ERG) parameters using 56 normal eyes of four dog breeds common in Thailand: poodle, Labrador retriever, Thai ridgeback, and Thai Bangkaew. Standard ERG findings were bilaterally recorded using a handheld multi-species ERG unit with an ERG-jet lens electrode for 28 dogs under preanesthesia with diazepam, anesthesia with propofol, and anesthesia maintenance with isoflurane. There were significant differences in the mean values of ERG amplitudes and implicit times among the four dog breeds (p < 0.05) except for the b-wave implicit time of the photopic 30 Hz flicker response with 3 cd.s/m2 (p = 0.610). Out of the four breeds, Thai Bangkaew had the longest implicit time (p < 0.001) of scotopic low intensity responses, b-wave of scotopic standard intensity responses (3 cd.s/m2), a-wave of the higher intensity response (10 cd.s/m2), and a-wave of the photopic single flash response (3 cd.s/m2). For the b/a ratio, only the ratio of the Cone response was significantly different among the different breeds. In this summary, normal ERG parameters for four dog breeds were reported. Data from the investigation supported the hypothesis that determination of breed-specific limits of normality for ERG responses is necessary for individual clinics and laboratories.
MeSH Terms
Figure
Reference
-
1. Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992; 110:1571–1576.2. Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993; 34:3278–3296.3. Ekesten B. Ophthalmic examination and diagnostics. In : Gelatt KN, editor. Veterinary Ophthalmology. 4th ed. Oxford: Wiley;2007. 1:p. 520–535.4. Ekesten B, Komáromy AM, Ofri R, Petersen-Jones SM, Narfström K. Guidelines of clinical electroretinography in the dog: 2012 update. Doc Ophthalmol. 2013; 127:79–87.
Article5. Elder MJ. Diazepam and its effects on visual fields. Aust N Z J Ophthalmol. 1992; 20:267–270.
Article6. Grozdanic SD, Kecova H, Harper MM, Nilaweera W, Kuehn MH, Kardon RH. Functional and structural changes in a canine model of hereditary primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2010; 51:255–263.
Article7. Gum GG, Gelatt KN, Samuelson DA. Maturation of the retina of the canine neonate as determined by electroretinography and histology. Am J Vet Res. 1984; 45:1166–1171.8. dos Santos Hansho C, Oria AP, da Veiga Monteiro Lazaro Júnior LP, Neto FD, Laus JL. The organization of flash electroretinography unit in veterinary medicine. Cienc Rural. 2004; 34:1097–1104.
Article9. Hertil E, Bergström T, Kell U, Karlstam L, Ekman S, Ekesten B. Retinal degeneration in nine Swedish Jämthund dogs. Vet Ophthalmol. 2010; 13:110–116.
Article10. Itoh Y, Maehara S, Yamasaki A, Tsuzuki K, Izumisawa Y. Investigation of fellow eye of unilateral retinal detachment in Shih-Tzu. Vet Ophthalmol. 2010; 13:289–293.
Article11. Jeong MB, Narfström K, Park SA, Chae JM, Seo KM. Comparison of the effects of three different combinations of general anesthetics on the electroretinogram of dogs. Doc Ophthalmol. 2009; 119:79–88.
Article12. Komáromy AM, Brooks DE, Dawson WW, Källberg ME, Ollivier FJ, Ofri R. Technical issues in electrodiagnositic recording. Vet Ophthalmol. 2002; 5:85–91.13. Kommonen B, Hyvätti E, Dawson WW. Propofol modulates inner retina function in Beagles. Vet Ophthalmol. 2007; 10:76–80.
Article14. Lin SL, Shiu WC, Liu PC, Cheng FP, Lin YC, Wang WS. The effects of different anesthetic agents on short electroretinography protocol in dogs. J Vet Med Sci. 2009; 71:763–768.
Article15. Maehara S, Itoh N, Wakaiki S, Yamasaki A, Tsuzuki K, Izumisawa Y. The effects of cataract stage, lens-induced uveitis and cataract removal on ERG in dogs with cataract. Vet Ophthalmol. 2007; 10:308–312.
Article16. Maehara S, Itoh N, Itoh Y, Wakaiki S, Tsuzuki K, Seno T, Kushiro T, Yamashita K, Izumisawa Y, Kotani T. Electroretinography using contact lens electrode with built-in light source in dogs. J Vet Med Sci. 2005; 67:509–514.
Article17. Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009; 118:69–77.
Article18. Mentzer AE, Eifler DM, Montiani-Ferreira F, Tuntivanich N, Forcier JQ, Petersen-Jones SM. Influence of recording electrode type and reference electrode position on the canine electroretinogram. Doc Ophthalmol. 2005; 111:95–106.
Article19. Mizota A, Adachi-Usami E. Effect of body temperature on electroretinogram of mice. Invest Ophthalmol Vis Sci. 2002; 43:3754–3757.20. Nair G, Kim M, Nagaoka T, Olson DE, Thulé PM, Pardue MT, Duong TQ. Effects of common anesthetics on eye movement and electroretinogram. Doc Ophthalmol. 2011; 122:163–176.
Article21. Narfstrom K. Electroretinography in veterinary medicine - easy or accurate? Vet Ophthalmol. 2002; 5:249–251.
Article22. Norman JC, Narfström K, Barrett PM. The effects of medetomidine hydrochloride on the electroretinogram of normal dogs. Vet Ophthalmol. 2008; 11:299–305.
Article23. Obasi CN, Barrett B, Brown R, Vrtis R, Barlow S, Muller D, Gern J. Detection of viral and bacterial pathogens in acute respiratory infections. J Infect. 2014; 68:125–130.
Article24. Ofri R. Retina. In : Maggs DJ, Miller PE, Ofri R, editors. Slatter's Fundamentals of Veterinary Ophthalmology. 4th ed. St. Louis: Saunders;2008. p. 285–295.25. Park SA, Yi NY, Jeong MB, Kim WT, Kim SE, Chae JM, Seo KM. Clinical manifestations of cataracts in small breed dogs. Vet Ophthalmol. 2009; 12:205–210.
Article26. Robbins J, Ikeda H. Benzodiazepines and the mammalian retina. I. Autorediographic localisation of receptor sites and the lack effect on the electroretinogram. Brain Res. 1989; 479:313–322.
Article27. Safatle AMV, Hvenegaard AP, Otsuki D, Martins TL, Kahvegian M, Berezovsky A, Salomão SR, Barros PSM. Comparison of full-field electroretinogram in diabetic and non diabetic dogs with cataracts. Pesqui Vet Bras. 2010; 30:1071–1076.
Article28. Stafanous SN, Clarke MP, Ashton H, Mitchell KW. The effect of long-term use of benzodiazepines on the eye and retina. Doc Ophthalmol. 1999; 99:55–68.29. Tuntivanich N, Mentzer AL, Eifler DM, Montiani-Ferreira F, Forcier JQ, Johnson CA, Petersen-Jones SM. Assessment of dark-adaptation time required for recovery of electroretinographic responses in dogs after fundus photography and indirect ophthalmoscopy. Am J Vet Res. 2005; 66:1798–1804.
Article30. Petersen-Jones SM. A review of research to elucidate the causes of the generalized progressive retinal atrophies. Vet J. 1998; 155:5–18.
Article31. Varela Lopez O, Alvarez Vazquez JC, Gonzalez Cantalapiedra A, Rosolen SG. Effect of hypercapnia on the electroretinogram in sevoflurane and isoflurane anaesthetized dogs. Doc Ophthalmol. 2010; 121:9–20.
Article32. Weleber RG. The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci. 1981; 20:392–399.33. Yanase J, Ogawa H, Ohtsuka H. Rod and cone components in the dog electroretinogram during and after dark adaptation. J Vet Med Sci. 1995; 57:877–881.
Article34. Zangerl B, Goldstein O, Philp AR, Lindauer SJP, Pearce-Kelling SE, Mullins RF, Graphodatsky AS, Ripoll D, Felix JS, Stone EM, Acland GM, Aguirre GD. Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics. 2006; 88:551–563.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of the Thai Version of the Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale
- Unfortunately, a “Back Light System†As a Global Positioning System Failed to Guide the Route in 25-G Fine-Needle Aspiration
- Acute-onset respiratory signs in a Labrador Retriever with a positive SARS-CoV-2 rapid antigen test and infection confirmed by RT-PCR analysis: a case report
- From Evidence to the Dish: A Viewpoint of Implementing a Thai-Style Mediterranean Diet for Parkinson’s Disease
- Incidence and Associated Factors of Infantile Colic in Thai Infants