J Vet Sci.
2015 Mar;16(1):37-46. 10.4142/jvs.2015.16.1.37.
Cloning, expression and functional analysis of the duck Toll-like receptor 5 (TLR5) gene
- Affiliations
-
- 1School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai Key Laboratory of Veterinary Biotechnology, Key Laboratory of Urban Agriculture (South), Ministry of Agriculture, Shanghai 200240, China. sunjhe@sjtu.edu.cn
- 2Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai 200241, China. shoveldeen@shvri.ac.cn
- 3Shandong Vocational Animal Science and Veterinary College, Weifang 261061, China.
- KMID: 2160819
- DOI: http://doi.org/10.4142/jvs.2015.16.1.37
Abstract
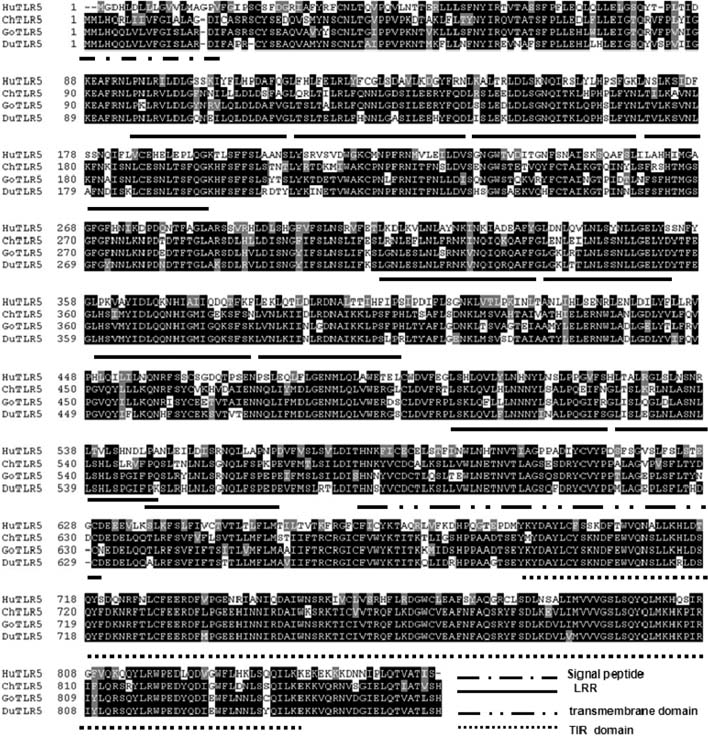
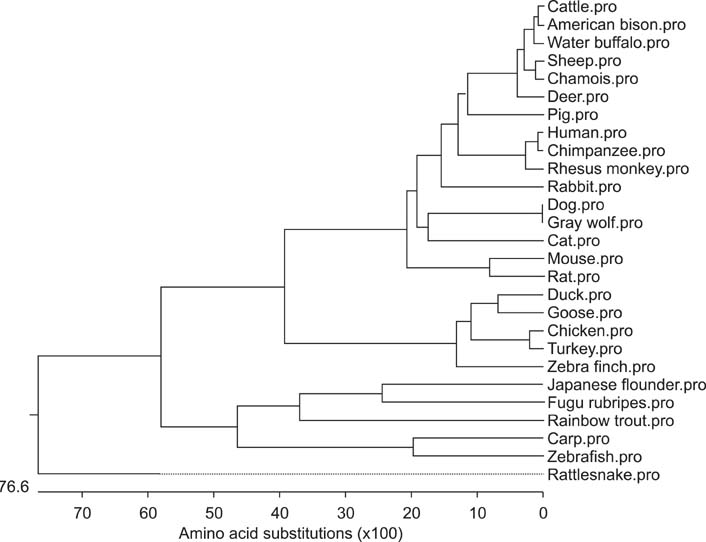
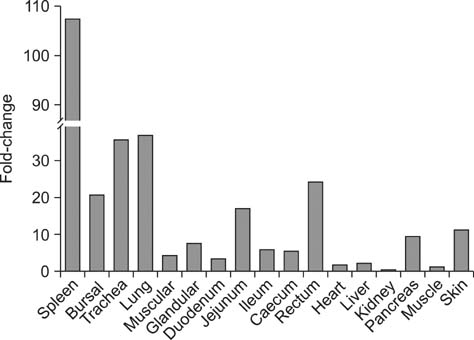
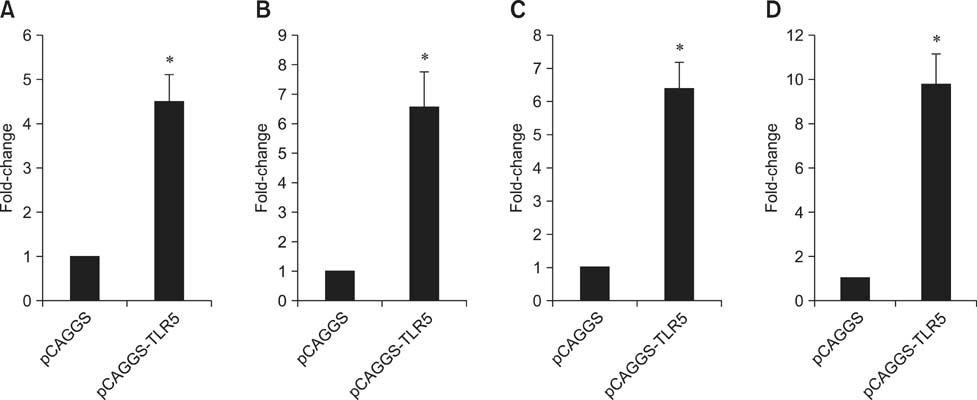
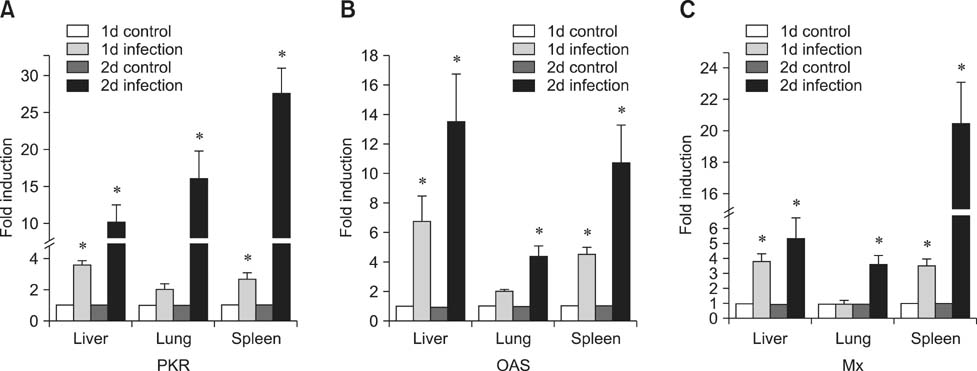
- Toll-like receptor 5 (TLR5) is responsible for the recognition of bacterial flagellin in vertebrates. In the present study, the first TLR5 gene in duck was cloned. The open reading frame (ORF) of duck TLR5 (dTLR5) cDNA is 2580 bp and encodes a polypeptide of 859 amino acids. We also cloned partial sequences of myeloid differentiation factor 88, 2'-5'-oligoadenylate synthetase (OAS), and myxovirus resistance (Mx) genes from duck. dTLR5 mRNA was highly expressed in the bursa of Fabricius, spleen, trachea, lung, jejunum, rectum, and skin; moderately expressed in the muscular and glandular tissues, duodenum, ileum, caecum, and pancreas; and minimally expressed in the heart, liver, kidney, and muscle. DF-1 or HeLa cells transfected with DNA constructs encoding dTLR5 can activate NF-kappaB leading to the activation of interleukin-6 (IL-6) promoter. When we challenged ducks with a Herts33 Newcastle disease virus (NDV), mRNA transcription of the antiviral molecules Mx, Double stranded RNA activated protein kinase (PKR), and OAS was up-regulated in the liver, lung, and spleen 1 and 2 days post-inoculation.
Keyword
MeSH Terms
-
2',5'-Oligoadenylate Synthetase/genetics/metabolism
Animals
Cell Line
*Cloning, Molecular
Ducks
Gene Expression Regulation/*physiology
Humans
Immunity, Innate
Myeloid Differentiation Factor 88/genetics/metabolism
Myxovirus Resistance Proteins/genetics/metabolism
Newcastle Disease/metabolism
Newcastle disease virus/classification
RNA, Messenger/genetics/metabolism
Species Specificity
Toll-Like Receptor 5/genetics/*metabolism
2',5'-Oligoadenylate Synthetase
Myeloid Differentiation Factor 88
Myxovirus Resistance Proteins
RNA, Messenger
Toll-Like Receptor 5
Figure
Reference
-
1. Barber MRW, Aldridge JR Jr, Webster RG, Magor KE. Association of RIG-I with innate immunity of ducks to influenza. Proc Natl Acad Sci U S A. 2010; 107:5913–5918.
Article2. Bernasconi D, Schultz U, Staeheli P. The interferon-induced Mx protein of chickens lacks antiviral activity. J Interferon Cytokine Res. 1995; 15:47–53.
Article3. Brownlie R, Allan B. Avian toll-like receptors. Cell Tissue Res. 2011; 343:121–130.
Article4. Chen S, Cheng A, Wang M. Innate sensing of viruses by pattern recognition receptors in birds. Vet Res. 2013; 44:82.
Article5. Daviet S, Van Borm S, Habyarimana A, Ahanda ML, Morin V, Oudin A, Van Den Berg T, Zoorob R. Induction of Mx and PKR failed to protect chickens from H5N1 infection. Viral Immunol. 2009; 22:467–472.
Article6. Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl JP, Sirard JC. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004; 172:6922–6930.
Article7. Fang Q, Pan Z, Geng S, Kang X, Huang J, Sun X, Li Q, Cai Y, Jiao X. Molecular cloning, characterization and expression of goose Toll-like receptor 5. Mol Immunol. 2012; 52:117–124.
Article8. Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001; 167:1882–1885.
Article9. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001; 410:1099–1103.
Article10. Huang Z, Chen X, Zhang K, Yu B, Mao X, Zhao Y, Chen D. Molecular cloning and functional characterization of Tibetan Porcine STING. Int J Mol Sci. 2012; 13:506–515.
Article11. Hug H, Costas M, Staeheli P, Aebi M, Weissmann C. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol Cell Biol. 1988; 8:3065–3079.
Article12. Ishibashi M, Wakita T, Esumi M. 2',5'-Oligoadenylate synthetase-like gene highly induced by hepatitis C virus infection in human liver is inhibitory to viral replication in vitro. Biochem Biophys Res Commun. 2010; 392:397–402.
Article13. Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009; 21:317–337.
Article14. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384.
Article15. Keestra AM, de Zoete MR, Bouwman LI, Vaezirad MM, van Putten JPM. Unique features of chicken Toll-like receptors. Dev Comp Immunol. 2013; 41:316–323.
Article16. Keestra AM, de Zoete MR, van Aubel RA, van Putten JPM. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol Immunol. 2008; 45:1298–1307.
Article17. Keestra AM, de Zoete MR, van Aubel RAMH, van Putten JP. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol Immunol. 2008; 45:1298–1307.
Article18. Khaperskyy DA, Hatchette TF, McCormick C. Influenza A virus inhibits cytoplasmic stress granule formation. FASEB J. 2012; 26:1629–1639.
Article19. Ko JH, Asano A, Kon Y, Watanabe T, Agui T. Characterization of the chicken PKR: polymorphism of the gene and antiviral activity against vesicular stomatitis virus. Jpn J Vet Res. 2004; 51:123–133.20. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23:2947–2948.
Article21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001; 25:402–408.
Article22. Marques J, Anwar J, Eskildsen-Larsen S, Rebouillat D, Paludan SR, Sen G, Williams BRG, Hartmann R. The p59 oligoadenylate synthetase-like protein possesses antiviral activity that requires the C-terminal ubiquitin-like domain. J Gen Virol. 2008; 89:2767–2772.
Article23. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001; 1:135–145.
Article24. Melchjorsen J, Kristiansen H, Christiansen R, Rintahaka J, Matikainen S, Paludan SR, Hartmann R. Differential regulation of the OASL and OAS1 genes in response to viral infections. J Interferon Cytokine Res. 2009; 29:199–207.25. Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009; 227:221–233.
Article26. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282:2085–2088.
Article27. Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005; 102:9577–9582.
Article28. Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009; 227:75–86.
Article29. Tatsumi R, Sekiya S, Nakanishi R, Mizutani M, Kojima S, Sokawa Y. Function of ubiquitin-like domain of chicken 2'-5'-oligoadenylate synthetase in conformational stability. J Interferon Cytokine Res. 2003; 23:667–676.
Article30. Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999; 401:811–815.
Article31. Vaheri A, Ruoslahti E, Hovi T, Nordling S. Stimulation of density-inhibited cell cultures by insulin. J Cell Physiol. 1973; 81:355–364.
Article32. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179.
Article33. Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, Tong L. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000; 408:111–115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decreased expression of Toll-like receptor 4 and 5 during progression of prostate transformation in transgenic adenocarcinoma of mouse prostate mice
- Association of Toll-Like Receptor 5 Gene Polymorphism with Susceptibility to Ossification of the Posterior Longitudinal Ligament of the Spine in Korean Population
- Cloning of Danio rerio toll-like receptor 1
- TLR5 Activation through NF-κB Is a Neuroprotective Mechanism of Postconditioning after Cerebral Ischemia in Mice
- Clinical Significance of Toll-Like Receptor and Toll-Like Receptor Blocker