Korean J Radiol.
2015 Oct;16(5):1056-1067. 10.3348/kjr.2015.16.5.1056.
Immunoglobulin G4-Related Kidney Disease: A Comprehensive Pictorial Review of the Imaging Spectrum, Mimickers, and Clinicopathological Characteristics
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. kimjhrad@amc.seoul.kr
- KMID: 2160773
- DOI: http://doi.org/10.3348/kjr.2015.16.5.1056
Abstract
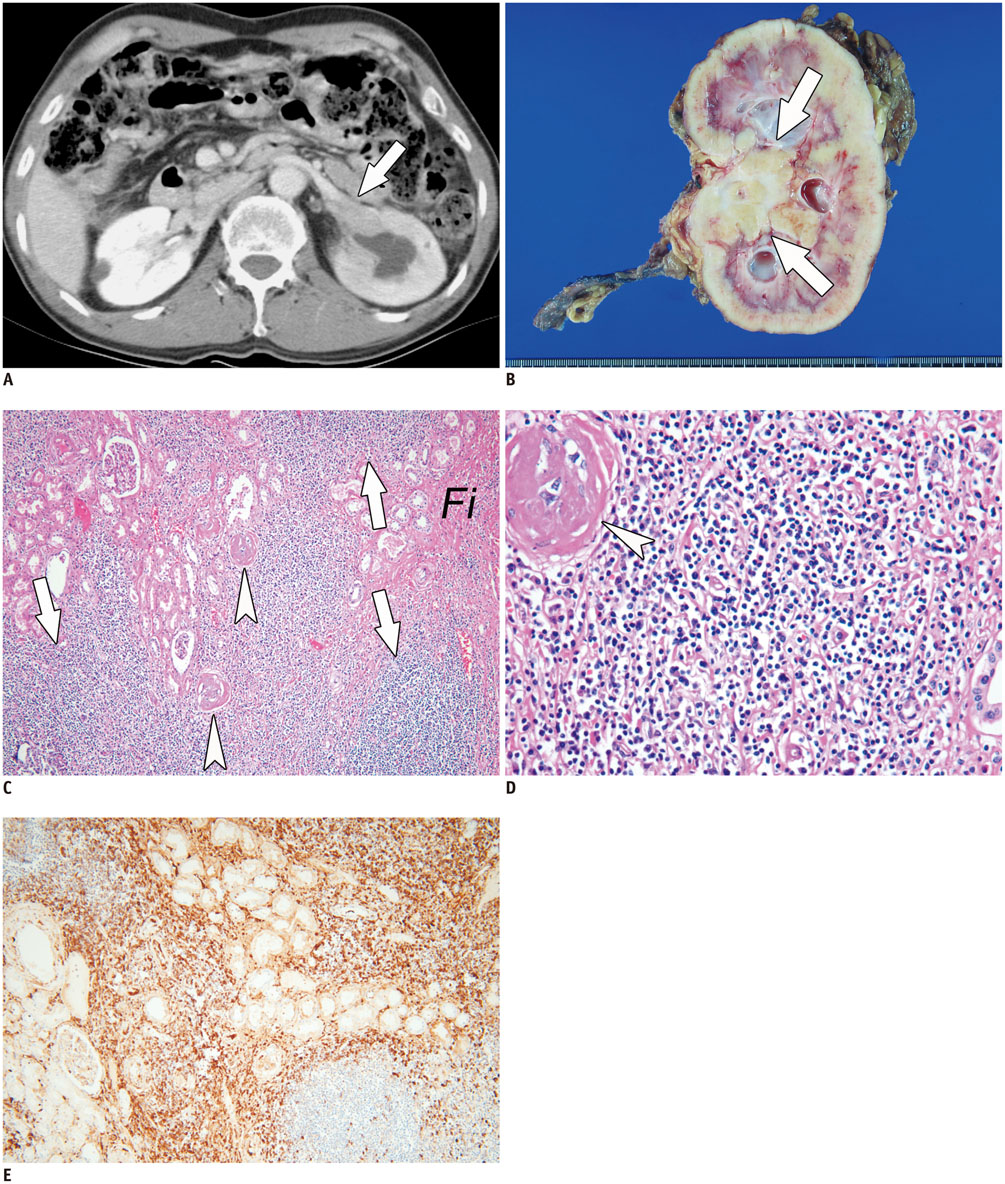
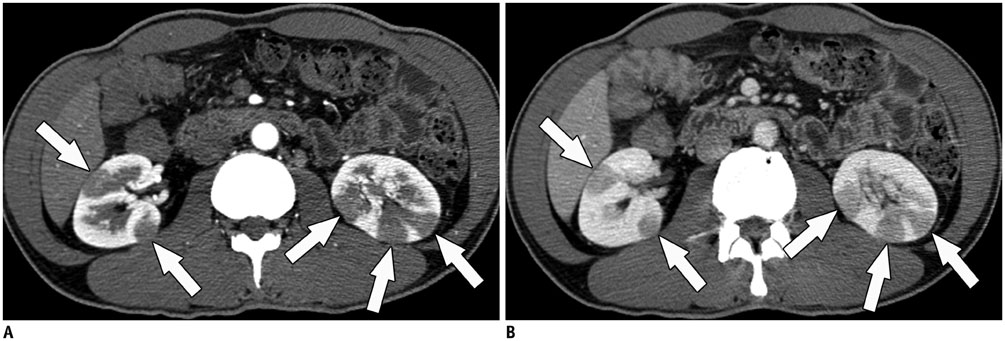
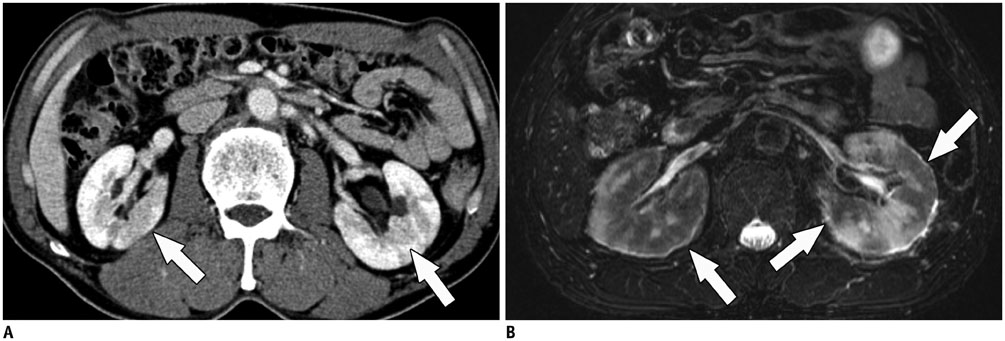
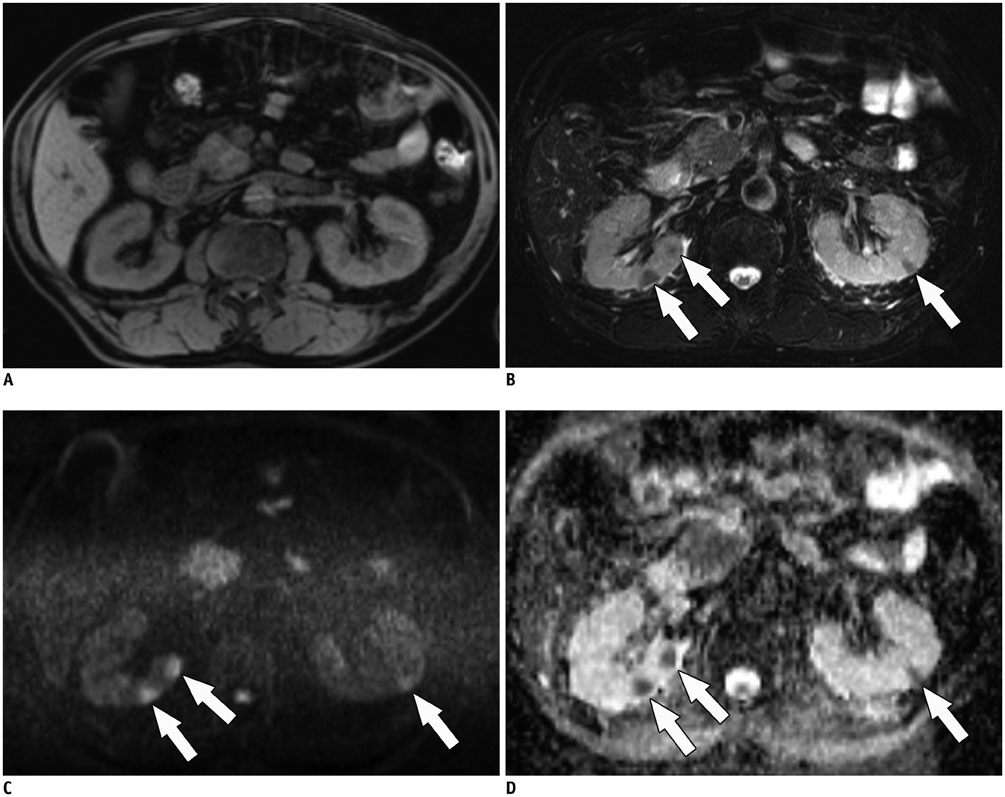
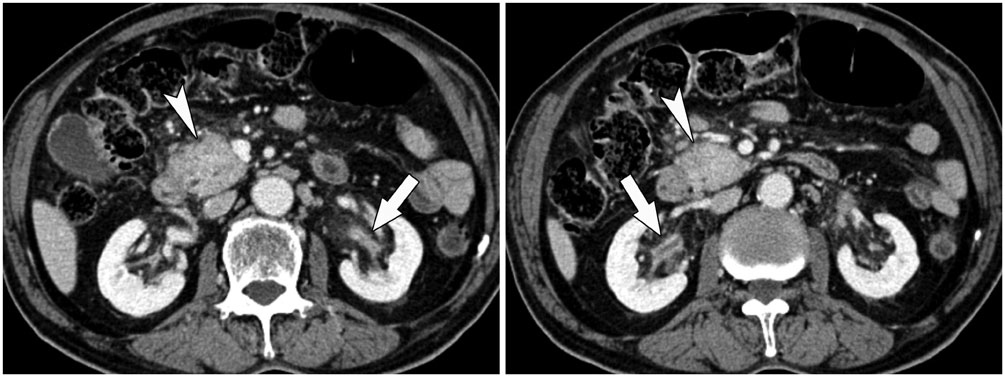
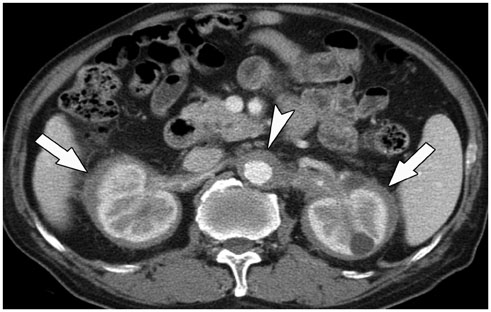
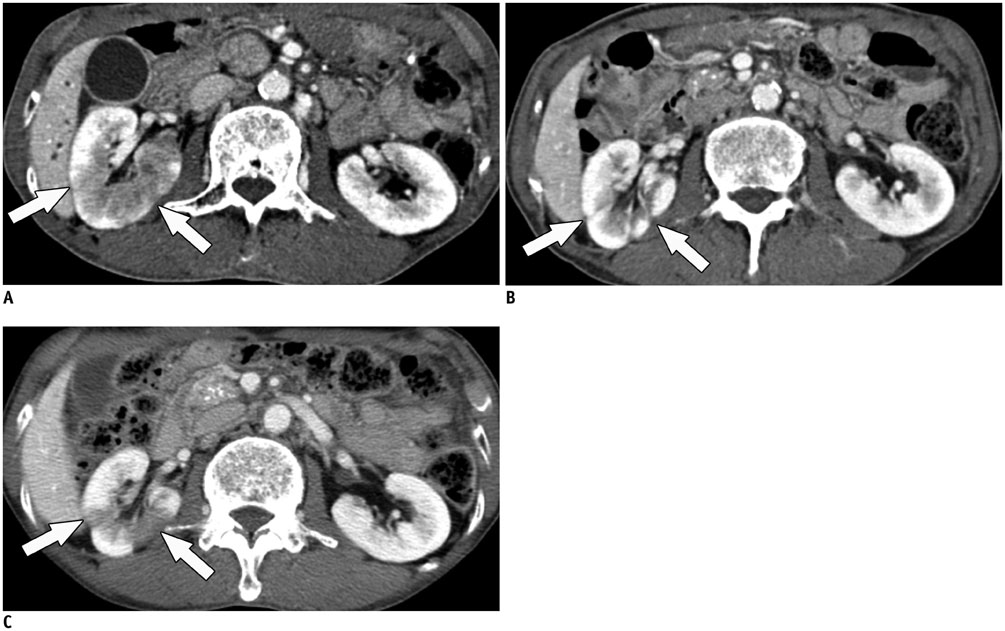
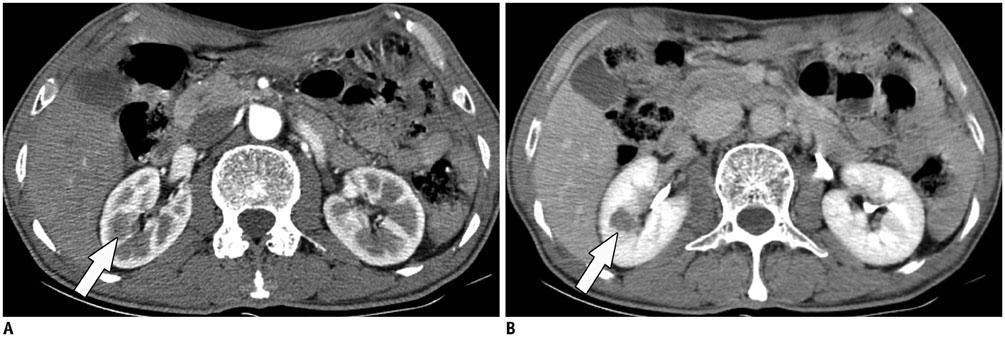
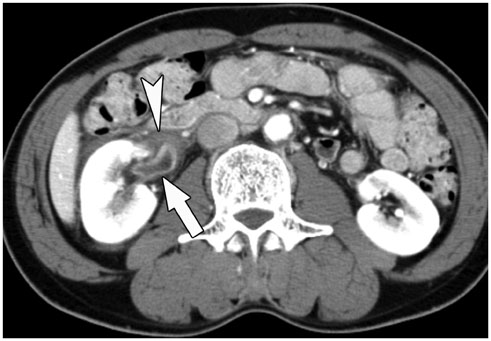
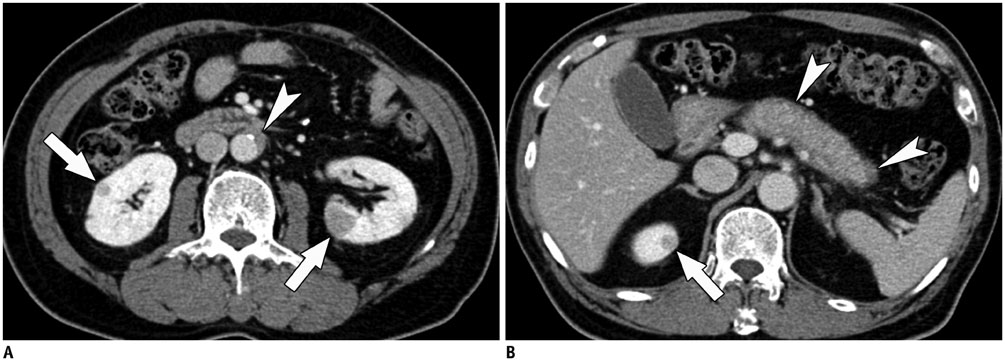
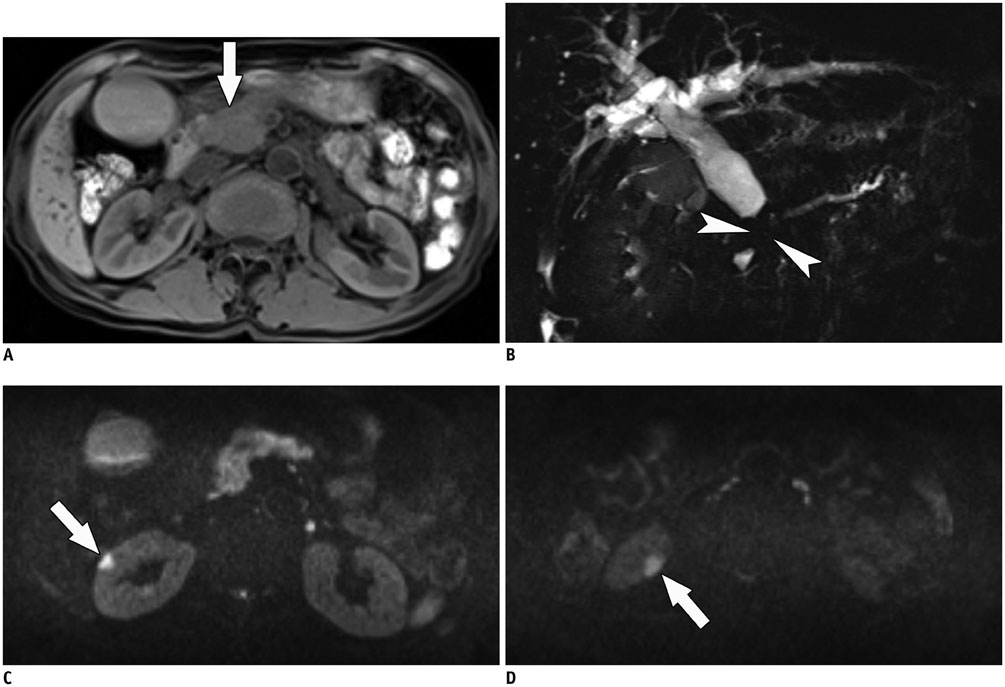
- Immunoglobulin G4 (IgG4)-related kidney disease (IgG4-KD) has recently been demonstrated to be an important part of IgG4-related sclerosing disease (IgG4-SD). However, since IgG4-KD is still relatively unfamiliar to radiologists and physicians as compared to IgG4-SD involving other organs, it could, therefore, be easily missed. In this article, we present a comprehensive pictorial review of IgG4-KD with regards to the imaging spectrum, mimickers, and clinicopathologic characteristics, based on our clinical experience with 48 patients during the past 13 years, as well as a literature review. Awareness of the broad imaging spectrum of IgG4-KD and differential diagnosis from its mimickers will thus facilitate its early diagnosis and treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Cornell LD. IgG4-related kidney disease. Semin Diagn Pathol. 2012; 29:245–250.2. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012; 366:539–551.3. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–1192.4. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012; 22:21–30.5. Raissian Y, Nasr SH, Larsen CP, Colvin RB, Smyrk TC, Takahashi N, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol. 2011; 22:1343–1352.6. Saeki T, Kawano M, Mizushima I, Yamamoto M, Wada Y, Nakashima H, et al. The clinical course of patients with IgG4-related kidney disease. Kidney Int. 2013; 84:826–833.7. Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011; 15:615–626.8. Khalili K, Doyle DJ, Chawla TP, Hanbidge AE. Renal cortical lesions in patients with autoimmune pancreatitis: a clue to differentiation from pancreatic malignancy. Eur J Radiol. 2008; 67:329–335.9. Kim B, Kim JH, Byun JH, Kim HJ, Lee SS, Kim SY, et al. IgG4-related kidney disease: MRI findings with emphasis on the usefulness of diffusion-weighted imaging. Eur J Radiol. 2014; 83:1057–1062.10. Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010; 78:1016–1023.11. Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol. 2011; 23:57–66.12. Takahashi N, Kawashima A, Fletcher JG, Chari ST. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology. 2007; 242:791–801.13. Kim JH, Kim MH, Byun JH, Lee SS, Lee SJ, Park SH, et al. Diagnostic strategy for differentiating autoimmune pancreatitis from pancreatic cancer: is an endoscopic retrograde pancreatography essential. Pancreas. 2012; 41:639–647.14. Kuroda N, Nakamura S, Miyazaki K, Inoue K, Ohara M, Mizuno K, et al. Chronic sclerosing pyelitis with an increased number of IgG4-positive plasma cells. Med Mol Morphol. 2009; 42:236–238.15. Kim SA, Lee SR, Huh J, Shen SS, Ro JY. IgG4-associated inflammatory pseudotumor of ureter: clinicopathologic and immunohistochemical study of 3 cases. Hum Pathol. 2011; 42:1178–1184.16. Sharma SG, Vlase HL, D'Agati VD. IgG4-related tubulointerstitial nephritis with plasma cell-rich renal arteritis. Am J Kidney Dis. 2013; 61:638–643.17. Saeki T, Kawano M. IgG4-related kidney disease. Kidney Int. 2014; 85:251–257.18. Yamaguchi Y, Kanetsuna Y, Honda K, Yamanaka N, Kawano M, Nagata M. Japanese study group on IgG4-related nephropathy. Characteristic tubulointerstitial nephritis in IgG4-related disease. Hum Pathol. 2012; 43:536–549.19. Triantopoulou C, Malachias G, Maniatis P, Anastopoulos J, Siafas I, Papailiou J. Renal lesions associated with autoimmune pancreatitis: CT findings. Acta Radiol. 2010; 51:702–707.20. Hedgire SS, McDermott S, Borczuk D, Elmi A, Saini S, Harisinghani MG. The spectrum of IgG4-related disease in the abdomen and pelvis. AJR Am J Roentgenol. 2013; 201:14–22.21. Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011; 31:1379–1402.22. Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Pozzi Mucelli R. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology. 2011; 260:428–436.23. Pozdzik AA, Matos C, Rorive S, Brocheriou I, Delhaye M, Nortier JL. Diffusion-weighted magnetic resonance imaging: a non-nephrotoxic prompt assessment of kidney involvement in IgG4-related disease. Kidney Int. 2014; 85:981.24. Sasiwimonphan K, Gorman B, Kawashima A, Chari ST, Takahashi N. Renal involvement in patients with autoimmune pancreatitis: ultrasound findings. Eur J Radiol. 2012; 81:807–810.25. Saunders HS, Dyer RB, Shifrin RY, Scharling ES, Bechtold RE, Zagoria RJ. The CT nephrogram: implications for evaluation of urinary tract disease. Radiographics. 1995; 15:1069–1085. discussion 1086-108826. Kawashima A, Sandler CM, Goldman SM, Raval BK, Fishman EK. CT of renal inflammatory disease. Radiographics. 1997; 17:851–866. discussion 867-86827. Suzer O, Shirkhoda A, Jafri SZ, Madrazo BL, Bis KG, Mastromatteo JF. CT features of renal infarction. Eur J Radiol. 2002; 44:59–64.28. Wong WS, Moss AA, Federle MP, Cochran ST, London SS. Renal infarction: CT diagnosis and correlation between CT findings and etiologies. Radiology. 1984; 150:201–205.29. Honda H, Coffman CE, Berbaum KS, Barloon TJ, Masuda K. CT analysis of metastatic neoplasms of the kidney. Comparison with primary renal cell carcinoma. Acta Radiol. 1992; 33:39–44.30. Bracken RB, Chica G, Johnson DE, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979; 72:806–807.31. Ganeshan D, Iyer R, Devine C, Bhosale P, Paulson E. Imaging of primary and secondary renal lymphoma. AJR Am J Roentgenol. 2013; 201:W712–W719.32. Jafri SZ, Bree RL, Amendola MA, Glazer GM, Schwab RE, Francis IR, et al. CT of renal and perirenal non-Hodgkin lymphoma. AJR Am J Roentgenol. 1982; 138:1101–1105.33. Semelka RC, Kelekis NL, Burdeny DA, Mitchell DG, Brown JJ, Siegelman ES. Renal lymphoma: demonstration by MR imaging. AJR Am J Roentgenol. 1996; 166:823–827.34. Saremi F, Knoll AN, Bendavid OJ, Schultze-Haakh H, Narula N, Sarlati F. Characterization of genitourinary lesions with diffusion-weighted imaging. Radiographics. 2009; 29:1295–1317.35. Yamashita Y, Takahashi M, Watanabe O, Yoshimatsu S, Ueno S, Ishimaru S, et al. Small renal cell carcinoma: pathologic and radiologic correlation. Radiology. 1992; 184:493–498.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systemic Manifestations of Immunoglobulin G4-Related Disease: A Pictorial Essay
- Overview of the Immunoglobulin G4-related Disease Spectrum
- Immunoglobulin G4-Related Systemic Sclerosing Disease: A Case Involving the Ureter and Kidney
- Immunoglobulin G4-Related Disease Involving Various Head and Neck Regions: A Case Report
- Immunoglobulin G4-related sclerosing cholangitis