Yonsei Med J.
2007 Aug;48(4):684-693. 10.3349/ymj.2007.48.4.684.
Heterogeneous Composition of Voltage-Dependent K+ Currents in Hepatic Stellate Cells
- Affiliations
-
- 1Division of Biobank for Health Sciences, Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Seoul, Korea. bokghee@nih.go.kr
- 2Center for Genome Sciences, Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Seoul, Korea.
- KMID: 2158176
- DOI: http://doi.org/10.3349/ymj.2007.48.4.684
Abstract
- PURPOSE
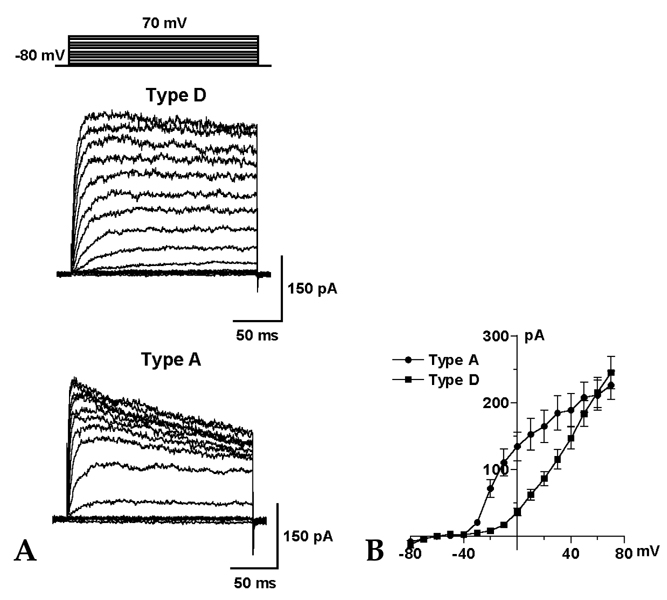
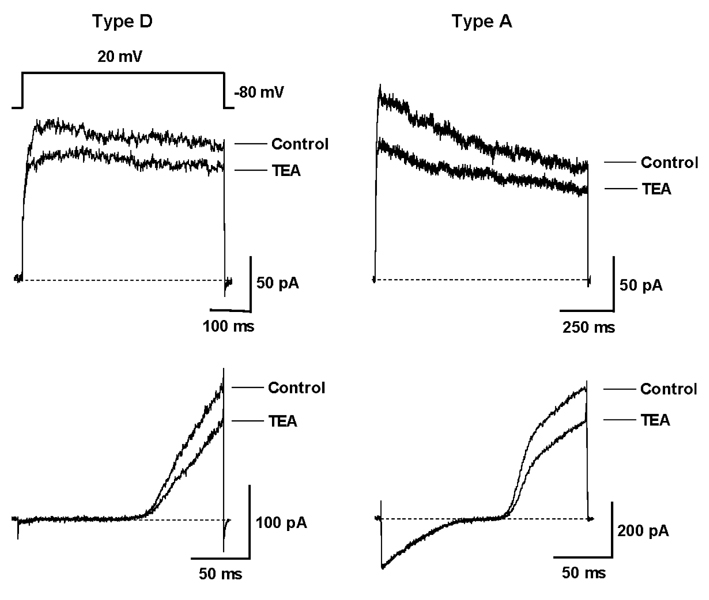
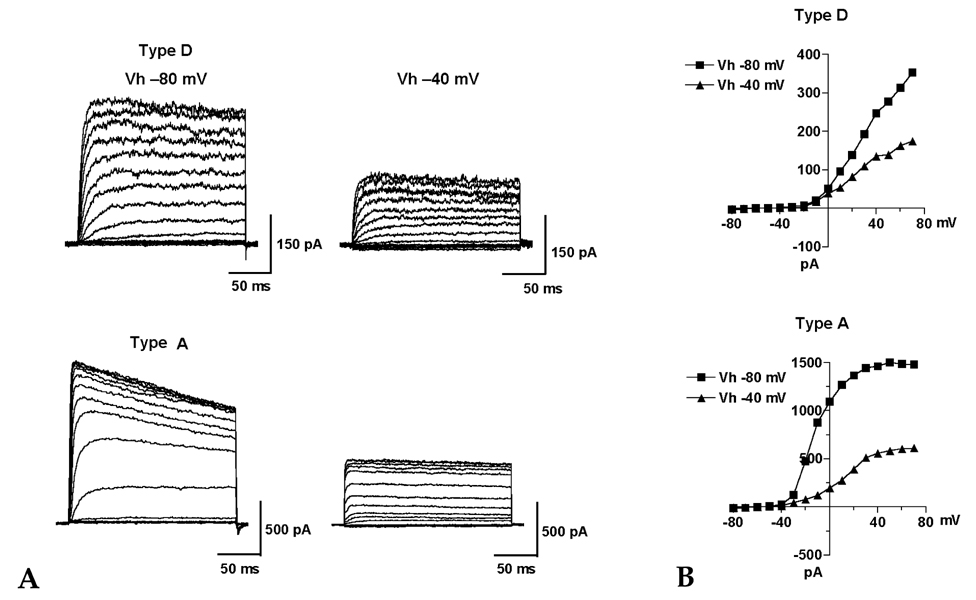
Hepatic stellate cells (HSC) are a type of pericyte with varying characteristics according to their location. However, the electrophysiological properties of HSC are not completely understood. Therefore, this study investigated the difference in the voltage-dependent K(+) currents in HSC. MATERIALS AND METHODS: The voltage-dependent K(+) currents in rat HSC were evaluated using the whole cell configuration of the patch-clamp technique. RESULTS: Four different types of voltage-dependent K(+) currents in HSC were identified based on the outward and inward K(+) currents. Type D had the dominant delayed rectifier K(+) current, and type A had the dominant transient outward K(+) current. Type I had an inwardly rectifying K(+) current, whereas the non-type I did not. TEA (5mM) and 4-AP (2mM) suppressed the outward K(+) currents differentially in type D and A. Changing the holding potential from -80 to -40mV reduced the amplitude of the transient outward K(+) currents in type A. The inwardly rectifying K(+) currents either declined markedly or were sustained in type I during the hyperpolarizing step pulses from -120 to -150mV. CONCLUSION: There are four different configurations of voltage-dependent K(+) currents expressed in cultured HSC. These results are expected to provide information that will help determine the properties of the K(+) currents in HSC as well as the different type HSC populations.
MeSH Terms
Figure
Cited by 1 articles
-
Changes in Inward Rectifier K+ Channels in Hepatic Stellate Cells During Primary Culture
Dong Hyeon Lee, In Deok Kong, Joong-Woo Lee, Kyu-Sang Park
Yonsei Med J. 2008;49(3):459-471. doi: 10.3349/ymj.2008.49.3.459.
Reference
-
1. Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996. 32:687–698.
Article2. Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000. 51:363–369.
Article3. Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993. 7:1031–1038.
Article4. Pinzani M. Novel insights into the biology and physiology of the Ito cell. Pharmacol Ther. 1995. 66:387–412.
Article5. Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993. 213:815–823.
Article6. Pinzani M, Failli P, Ruocco C, Casini A, Milani S, Baldi E, et al. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992. 90:642–646.
Article7. Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997. 430:195–207.
Article8. Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, et al. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999. 154:153–167.
Article9. Ballardini G, Fallani M, Biagini G, Bianchi FB, Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988. 56:45–49.
Article10. Ballardini G, Groff P, Badiali de Giorgi L, Schuppan D, Bianchi FB. Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology. 1994. 19:440–446.
Article11. Wake K, Sato T. Intralobular heterogeneity of perisinusoidal stellate cells in porcine liver. Cell Tissue Res. 1993. 273:227–237.
Article12. Zhang Z, Rhinehart K, Pallone TL. Membrane potential controls calcium entry into descending vasa recta pericytes. Am J Physiol Regul Integr Comp Physiol. 2002. 283:R949–R957.13. Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol. 1999. 521:637–650.
Article14. von Beckerath N, Nees S, Neumann FJ, Krebs B, Juchem G, Schömig A. An inward rectifier and a voltage-dependent K+ current in single, cultured pericytes from bovine heart. Cardiovasc Res. 2000. 46:569–578.
Article15. Lee KI, Kong ID, Baik SK, Kim HS, Lee DK, Kwon SO, et al. Characteristics of potassium and calcium currents of hepatic stellate cells (ito) in rat. Yonsei Med J. 2004. 45:649–660.
Article16. Kashiwagi S, Suematsu M, Wakabayashi Y, Kawada N, Tachibana M, Koizumi A, et al. Electrophysiological characterization of cultured hepatic stellate cells in rats. Am J Physiol. 1997. 272:G742–G750.
Article17. Gasull X, Batallr R, Ginès P, Sancho-Bru P, Nicolás JM, Görbig MN, et al. Human myofibroblastic hepatic stellate cells express Ca2+-activated K+ channels that modulate the effects of endothelin-1 and nitric oxide. J Hepatol. 2001. 35:739–748.
Article18. Bataller R, Gasull X, Ginès P, Hellemans K, Görbig MN, Nicols JM, et al. In vitro and in vivo activation of rat hepatic stellate cells results in de novo expression of L-type voltage-operated calcium channels. Hepatology. 2001. 33:956–962.
Article19. Bedossa P, Houglum K, Trautwein C, Holstege A, Chojkier M. Stimulation of collagen alpha 1(I) gene expression is associated with lipid peroxidation in hepatocellular injury: a link to tissue fibrosis? Hepatology. 1994. 19:1262–1271.
Article20. Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, et al. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984. 4:709–714.
Article21. Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992. 24:193–203.22. Hille B. Ionic Channels of Excitable Membranes. 1992. Sunderland, MA: Sinauer.23. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995. 268:C799–C822.
Article24. Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988. 404:455–466.
Article25. Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+. Am J Physiol. 1996. 271:H696–H705.26. Snetkov VA, Ward JP. Ion currents in smooth muscle cells from human small bronchioles: presence of an inward rectifier K+ current and three types of large conductance K+ channel. Exp Physiol. 1999. 84:835–846.
Article27. Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989. 69:546–604.
Article28. Biermans G, Vereecke J, Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987. 410:604–613.
Article29. Xu X, Rials SJ, Wu Y, Marinchak RA, Kowey PR. The properties of the inward rectifier potassium currents in rabbit coronary arterial smooth muscle cells. Pflugers Arch. 1999. 438:187–194.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Potassium and Calcium Currents of Hepatic Stellate Cells (Ito) in Rat
- Effects of Tamoxifen on the Voltage-dependent Ionic Currents in Mouse Colonic Smooth Muscle Cells
- Depolarizing Mechanisms of Acetylcholine in Gastric Smooth Muscle
- Effects of Propofol in Voltage-dependent Potassium Channels in Human Neurl Stem Cells
- Effects of acidic pH on voltage-gated ion channels in rat trigeminal mesencephalic nucleus neurons