Yonsei Med J.
2005 Feb;46(1):51-60. 10.3349/ymj.2005.46.1.51.
Therapeutic Effects of Holmium-166 Chitosan Complex in Rat Brain Tumor Model
- Affiliations
-
- 1Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea. jchang@yumc.yonsei.ac.kr
- 2Department of Nuclear Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Neurosurgery, Pochun CHA University College of Medicine, Sungnam, Korea.
- KMID: 2158112
- DOI: http://doi.org/10.3349/ymj.2005.46.1.51
Abstract
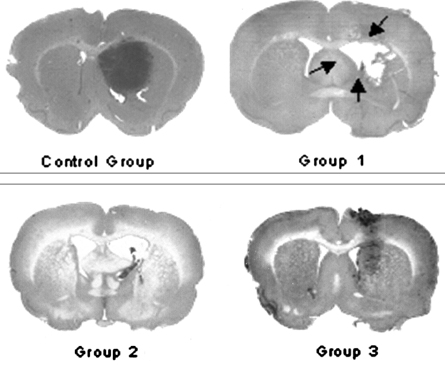
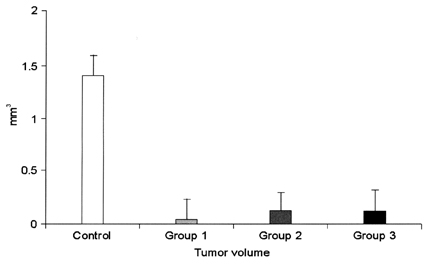
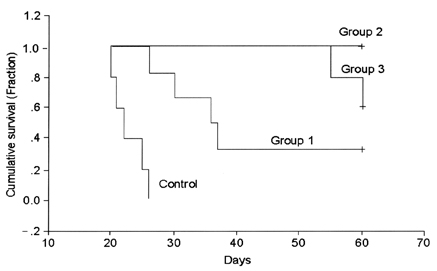
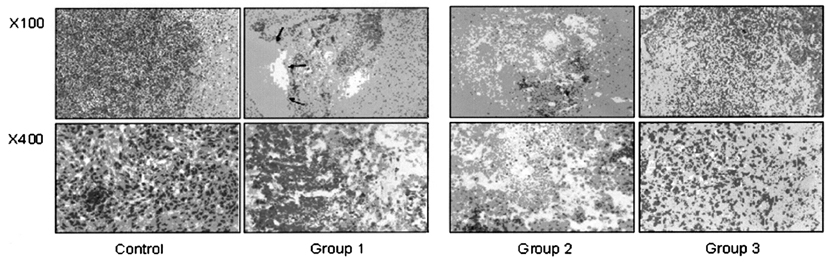
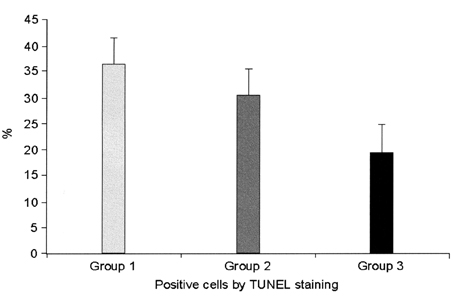
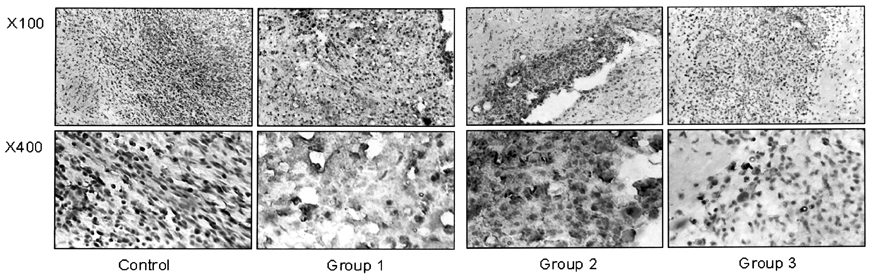
- This study examined the effectiveness of Holmium-166 (Ho-166) chitosan complex therapy for a malignant glioma. Cultured C6 glioma cells (100, 000 in 5microliter) were injected into the caudate/putamen of 200 - 250 gram Wistar rats. Five days later, a Ho-166 chitosan complex was injected into the same site of the glioma injection. Four injection doses were administered: the control group received PBS 10microliter, group 1 received an injection of 100micro Ci (10microliter), group 2 received an injection of 50microCi (5microliter), and group 3 received an injection of 10micro Ci (1microliter). The average tumor volume for each group was 1.385 mm3 for the control group, 0.036 mm3 for group 1, 0.104 mm3 for group 2, and 0.111 mm3 for group 3. Compared with the control group, the size of the tumors in groups 1, 2 and 3 was reduced by an average of 97.4%, 92.5% and 91.9%, respectively. The Kaplan-Meier survival curve of group 2 was the longest, followed by groups 3, group 1 and the control. The mean survival was 22.8, 59, 60, and 44.6 days for the control group and groups 3, 2 and 1, respectively. H-E staining revealed that group 2 yielded the best results in the destruction of the malignant glioma. TUNEL staining and immunohistochemical studies indicated apoptotic features. The Ho-166 chitosan complex proved to be effective in destroying the malignant glioma.
MeSH Terms
-
Animals
Brachytherapy
Brain Neoplasms/mortality/pathology/*radionuclide imaging
Cell Line, Tumor
Chitin/analogs & derivatives/*pharmacology
Disease Models, Animal
Glioma/mortality/pathology/*radionuclide imaging
Holmium/*pharmacology
Radioisotopes/*pharmacology
Rats
Rats, Wistar
Research Support, Non-U.S. Gov't
Figure
Reference
-
1. Chang JW, Lee H, Kim E, Lee Y, Chung SS, Kim JH. Combined antitumor effects of an Adenoviral Cytosine Deaminase/Thymidine Kinase fusion gene in rat c6 glioma. Neurosurgery. 2000. 47:931–939.2. Brandes A, Soesan M, Fiorentino MV. Medical treatment of high grade malignant glioma in adults: An overview. Anticancer Res. 1991. 11:719–727.3. Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000. 26:397–409.4. Bampoe J, Glen J, Hubbard SL, Salhia B, Shannon P, Rutka J, et al. Adenoviral vector-mediated gene transfer: timing of wild-type p53 gene expression in vivo and effect of tumor transduction on survival in a rat glioma brachytherapy model. J Neurooncol. 2000. 49:27–39.5. Bampoe J, Laperriere N, Pintilie M, Glen J, Micallef J, Bernstein M. Quality of life in patients with glioblastoma multiforme participating in a randomized study of brachytherapy as a boost treatment. J Neurosurg. 2000. 93:917–926.6. Patel S, Breneman JC, Warnick RE, Albright RE Jr, Tobler WD, van Loveren HR, et al. Permanent iodine-125 interstitial implants for the treatment of recurrent glioblastoma multiforme. Neurosurgery. 2000. 46:1123–1130.7. Oppenheimer JH, Levy ML, Sinha U, El-Kadi H, Apuzzo ML, Luxton G, et al. Radionecrosis secondary to interstitial brachytherapy: correlation of magnetic resonance imaging and histopathology. Neurosurgery. 1992. 31:336–343.8. Houston SC, Crocker IR, Brat DJ, Olson JJ. Extraneural metastatic glioblastoma after interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2000. 48:831–836.9. Mumper RJ, Ryo UY, Jay M. Neutron-Activated Holmium-166-Poly (L-Lactic Acid) Microspheres: A Potential Agent for the Internal Radiation Therapy of Hepatic Tumors. J Nucl Med. 1991. 32:2139–2143.10. Chung YL, Lee JD, Bang D, Lee JB, Park KB, Lee MG. Treatment of Bowen's disease with a specially designed radioactive skin patch. Eur J Nucl Med. 2000. 27:842–846.11. Lee JD, Park KB, Lee MG, Kim EH, Rhim KJ, Lee JT, et al. Radionuclide Therapy of Skin Cancers and Bowen's Disease Using a Specially Designed Skin Patch. J Nucl Med. 1997. 38:697–702.12. Lee JD, Yang WI, Lee MG, Ryu YH, Park JH, Shin KH, et al. Effective local control of malignant melanoma by intratumoral injection of a beta-emitting radionuclide. Eur J Nucl Med. 2002. 29:221–230.13. Vila A, Sanchez A, Janes K, Behrens I, Kissel T, Jato JL, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004. 57:123–131.14. Lee WY, Moon EY, Lee J, Choi CH, Nam SC, Park KB, et al. Toxicities of 166 Holmium-chitosan in mice. Arzneimittelforschung. 1998. 48:300–304.15. Bradford R, Darling JL, Thomas DGT. The development of an animal model of glioma for use in experimental neurooncology. Br J Neurosurg. 1989. 3:197–210.16. Engebraaten O, Hjortland GO, Hirschberg H, Fodstad O. Growth of precultured human glioma specimens in nude rat brain. J Neurosurg. 1999. 90:125–132.17. Chandler KL, Prados MD, Malec M, Wilson CB. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993. 32:716–720.18. Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987. 21:201–206.19. Levin VA. Chemotherapy for brain tumors of astrocytic and oligodendroglial lineage: the past decade and where we are heading. Neuro-oncol. 1999. 1:69–80.20. Nwokedi EC, DiBiase SJ, Jabbour S, Herman J, Amin P, Chin LS. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002. 50:41–47.21. Park YG, Chung SS, Kim DI, Chang JW, Cho J. Complications following Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 1995. 64:Suppl 1. 239–248.22. Kureshi SA, Hofman FM, Schneider JH, Chin LS, Apuzzo ML, Hinton DR. Cytokine expression in radiation-induced delayed cerebral injury. Neurosurgery. 1994. 35:822–830.23. Plowman PN. Stereotactic radiosurgery. VIII. The classification of postradiation reactions. Br J Neurosurg. 1999. 13:256–264.24. Lee YH. Effect of Holmium-166 Injection into Hepatocellular Carcinomas (SK-HEP1) Heterotransplanted in Mice. Journal of the Korean Radiological Society. 1998. 38:83–92.25. Song J, Suh CH, Park YB, Lee SH, Yoo NC, Lee JD, et al. A phase I/IIa study on intra- articular injection of Holmium-166 chitosan complex for the treatment of knee synovitis of rheumatoid arthritis. Eur J Nucl Med. 2001. 28:489–497.26. Suzuki YS, Momose Y, Higashi N, Shigematsu A, Park KB, Kim YM, et al. Biodistribution and kinetics of Holmium-166-chitosan complexes in rats and mice. J Nucl Med. 1998. 39:2161–2166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preclinical Trial of Radiation Synovectomy with Ho-166
- Percutaneous Sclerotherapy of Renal Cysts with a Beta-Emitting Radionuclide, Holmium-166-chitosan Complex
- Intracavitary Radiation Therapy for Recurrent Cystic Brain Tumors with Holmium-166-Chico : A Pilot Study
- Beta Dosimetry for Applying 166Ho-chitosan Complex to Cystic Brain Tumor Treatment: Monte Carlo Simulations Using a Spherical Model
- 166Ho - chitosan as a radiation synovectomy agent - Biocompatibility study of 166Ho - chitosan in rabbits