J Korean Med Sci.
2011 Mar;26(3):379-385. 10.3346/jkms.2011.26.3.379.
Predictors of Pulmonary Function Response to Treatment with Salmeterol/fluticasone in Patients with Chronic Obstructive Pulmonary Disease
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Asthma Center, and Clinical Research Center for Chronic Obstructive Airway Diseases, University of Ulsan College of Medicine, Seoul, Korea. sdlee@amc.seoul.kr
- 2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pulmonary and Critical Care Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 4Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Bundang CHA Hospital, College of Medicine, CHA University, Seongnam, Korea.
- 5Department of Radiology, East-West Neo Medical Center, Kyunghee University, Seoul, Korea.
- 6Division of Pulmonology, Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
- 7Department of Internal Medicine, College of Medicine, Kangwon National University, Chuncheon, Korea.
- 8Department of Internal Medicine, Ewha Womens University Mokdong Hospital, College of Medicine, Ewha Womans University, Seoul, Korea.
- 9Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University College of Medicine, Clinical Research Institute, Seoul, Korea.
- 10Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, College of Medicine, Korea University Anam Hospital, Seoul, Korea.
- 11Division of Pulmonary and Critical Care Medicine, Department of Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 13Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 14Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2157875
- DOI: http://doi.org/10.3346/jkms.2011.26.3.379
Abstract
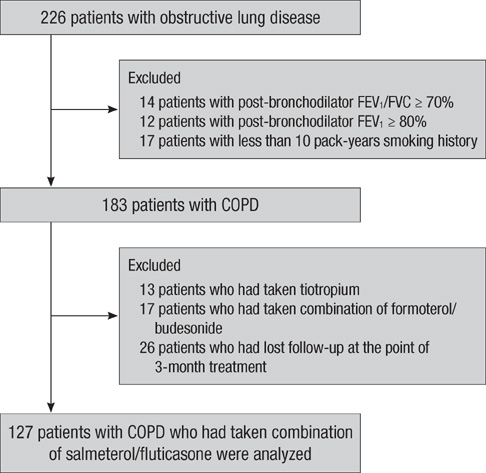
- Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease and responses to therapies are highly variable. The aim of this study was to identify the predictors of pulmonary function response to 3 months of treatment with salmeterol/fluticasone in patients with COPD. A total of 127 patients with stable COPD from the Korean Obstructive Lung Disease (KOLD) Cohort, which were prospectively recruited from June 2005 to September 2009, were analyzed retrospectively. The prediction models for the FEV1, FVC and IC/TLC changes after 3 months of treatment with salmeterol/fluticasone were constructed by using multiple, stepwise, linear regression analysis. The prediction model for the FEV1 change after 3 months of treatment included wheezing history, pre-bronchodilator FEV1, post-bronchodilator FEV1 change and emphysema extent on CT (R = 0.578). The prediction models for the FVC change after 3 months of treatment included pre-bronchodilator FVC, post-bronchodilator FVC change (R = 0.533), and those of IC/ TLC change after 3 months of treatment did pre-bronchodilator IC/TLC and post-bronchodilator FEV1 change (R = 0.401). Wheezing history, pre-bronchodilator pulmonary function, bronchodilator responsiveness, and emphysema extent may be used for predicting the pulmonary function response to 3 months of treatment with salmeterol/fluticasone in patients with COPD.
Keyword
MeSH Terms
-
Aged
Albuterol/*analogs & derivatives/therapeutic use
Androstadienes/*therapeutic use
Bronchodilator Agents/*therapeutic use
Emphysema
Female
Humans
Linear Models
Lung/physiopathology
Male
Middle Aged
Prognosis
Pulmonary Disease, Chronic Obstructive/*drug therapy
Republic of Korea
Respiratory Function Tests
Retrospective Studies
Tomography Scanners, X-Ray Computed
Treatment Outcome
Figure
Cited by 1 articles
-
Efficacy and Safety of Roflumilast in Korean Patients with COPD
Jae Seung Lee, Yoon Ki Hong, Tae Sun Park, Sei Won Lee, Yeon-Mok Oh, Sang-Do Lee
Yonsei Med J. 2016;57(4):928-935. doi: 10.3349/ymj.2016.57.4.928.
Reference
-
1. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007. 176:532–555.2. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000. 320:1297–1303.3. Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000. 343:1902–1909.4. Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002. 166:1084–1091.5. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007. 356:775–789.6. The COPD guidelines group of the standards of care committee of the BTS. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax. 1997. 52:Suppl 5. S1–S28.7. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA. Prednisolone response in patients with chronic obstructive pulmonary disease: results from the ISOLDE study. Thorax. 2003. 58:654–658.8. Kim WJ, Oh YM, Sung J, Kim TH, Huh JW, Jung H, Lee JH, Kim EK, Lee JH, Lee SM, Lee S, Lim SY, Shin TR, Yoon HI, Kwon SY, Lee SD. Lung function response to 12-week treatment with combined inhalation of long-acting beta2 agonist and glucocorticoid according to ADRB2 polymorphism in patients with chronic obstructive pulmonary disease. Lung. 2008. 186:381–386.9. Terminology, definitions, and classification of chronic pulmonary emphysema and related conditions: a report of the conclusions of a Ciba guest symposium. Thorax. 1959. 14:286–299.10. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, Strachan D, Weiland SK, Williams HC. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995. 8:483–491.11. Park JO, Choi IS, Park KO. Normal predicted standards of single breath carbon monoxide diffusing capacity of lung in healthy nonsmoking adults. Korean J Intern Med. 1985. 28:176–183.12. Choi JK, Paek D, Lee JO. Normal predicted values of spirometry in Korean population. Tuberc Respir Dis. 2005. 58:230–242.13. Quanjer P, Dalhuijsen A, Van Zoramen B. Standardized lung function testing. Report of the working party for the European Community for Coal and Steel. Bull Eur Physiopathol Respir. 1983. 19:Suppl 5. 1–95.14. Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, Seo JB, Lee SD. KOLD Study Group. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008. 186:157–165.15. Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995. 152:653–657.16. Wood SA, Zerhouni EA, Hoford JD, Hoffman EA, Mitzner W. Measurement of three-dimensional lung tree structures by using computed tomography. J Appl Physiol. 1995. 79:1687–1697.17. Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003. 58:659–664.18. Calverley P, Pauwels RA, Jones PW, Anderson JA, Vestbos J. The severity of airways obstruction as a determinant of treatment response in COPD. Int J Chron Obstruct Pulmon Dis. 2006. 1:209–218.19. Jenkins CR, Jones PW, Calverley PM, Celli B, Anderson JA, Ferguson GT, Yates JC, Willits LR, Vestbo J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009. 10:59.20. Meslier N, Charbonneau G, Racineux J. Wheezes. Eur Respir J. 1995. 8:1942–1948.21. Marini JJ, Pierson DJ, Hudson LD, Lakshminarayan S. The significance of wheezing in chronic airflow obstruction. Am Rev Respir Dis. 1979. 120:1069–1072.22. Chanez P, Vignola AM, O'Shaugnessy T, Enander I, Li D, Jeffery PK, Bousquet J. Corticosteroid reversibility in COPD is related to features of asthma. Am J Respir Crit Care Med. 1997. 155:1529–1534.23. Kitaguchi Y, Fujimoto K, Kubo K, Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med. 2006. 100:1742–1752.24. Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000. 162:1102–1108.25. Boschetto P, Miniati M, Miotto D, Braccioni F, De Rosa E, Bononi I, Papi A, Saetta M, Fabbri LM, Mapp CE. Predominant emphysema phenotype in chronic obstructive pulmonary. Eur Respir J. 2003. 21:450–454.26. Makita H, Nasuhara Y, Nagai K, Ito Y, Hasegawa M, Betsuyaku T, Onodera Y, Hizawa N, Nishimura M. Hokkaido COPD Cohort Study Group. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007. 62:932–937.27. Lee JH, Lee YK, Kim EK, Kim TH, Huh JW, Kim WJ, Lee JH, Lee SM, Lee S, Lim SY, Shin TR, Yoon HI, Sheen SS, Kim N, Seo JB, Oh YM, Lee SD. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med. 2010. 104:542–549.28. Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Paré PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005. 171:142–146.29. Lapperre TS, Snoeck-Stroband JB, Gosman MM, Stolk J, Sont JK, Jansen DF, Kerstjens HA, Postma DS, Sterk PJ. Groningen and Leiden Universities Corticosteroids in Obstructive Lung Disease Study Group. Dissociation of lung function and airway inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004. 170:499–504.30. Han MK, Postma D, Mannino DM, Giardino ND, Buist S, Curtis JL, Martinez FJ. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007. 176:1179–1184.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetic characteristics of fluticasone, salmeterol and tiotropium after concurrent inhalation
- Cor Pulmonale with Particular Reference to Chronic Obstructive Pulmonary Disease and Pulmonary Tuberculosis
- The Study on the Effects of a Respiratory Rehabilitation Program for COPD Patients
- Comparison for the Effects of Triple Therapy with Salmeterol/Fluticasone Propionate and Tiotropium Bromide versus Individual Components in Patients of Severe COPD Combined with Bronchial Hyperresponsiveness
- Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease (COPD)