J Korean Med Sci.
2010 Oct;25(10):1411-1417. 10.3346/jkms.2010.25.10.1411.
Inhibition of Hypoxic Pulmonary Vasoconstriction of Rats by Carbon Monoxide
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul, Korea. sjoonkim@snu.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2157842
- DOI: http://doi.org/10.3346/jkms.2010.25.10.1411
Abstract
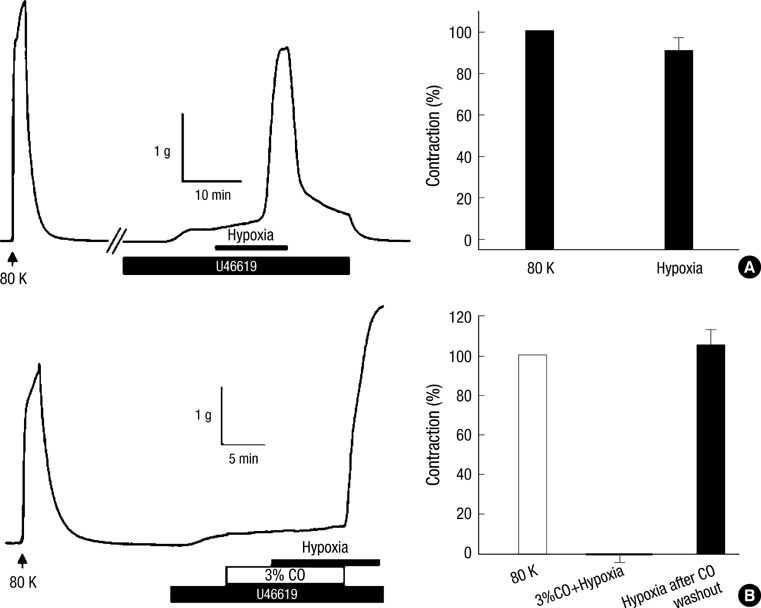
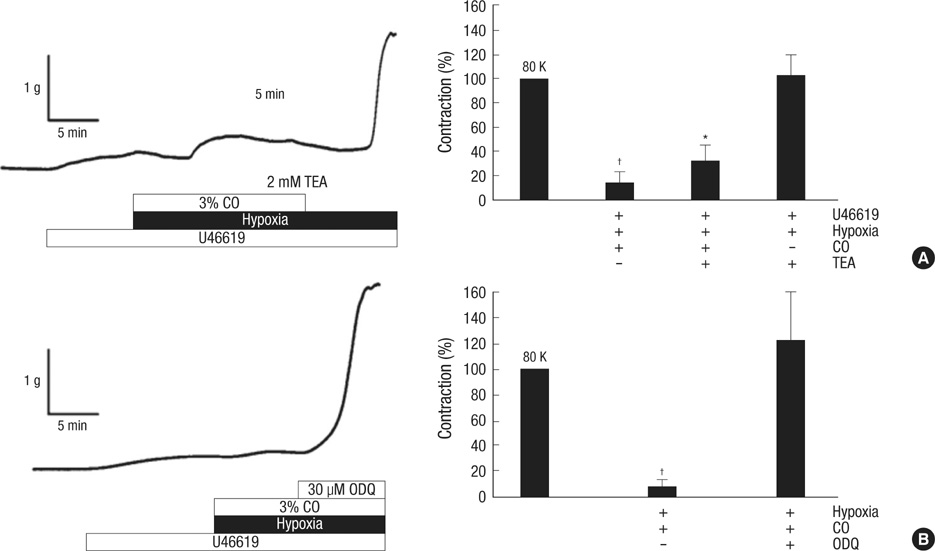
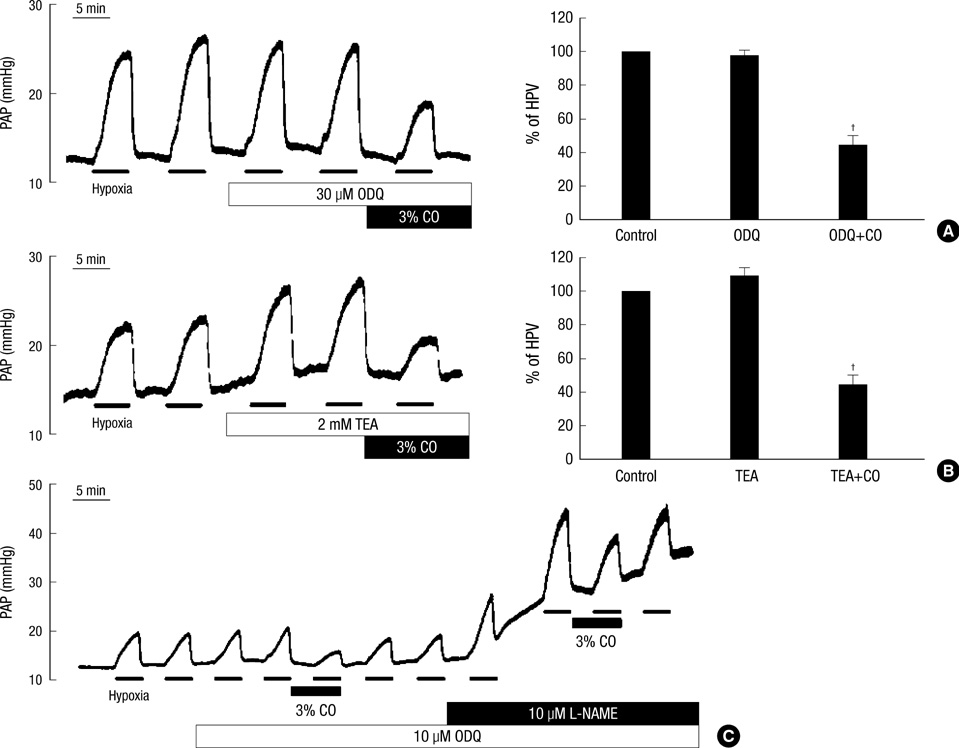
- Hypoxic pulmonary vasoconstriction (HPV), a unique response of pulmonary circulation, is critical to prevent hypoxemia under local hypoventilation. Hypoxic inhibition of K+ channel is known as an important O2-sensing mechanism in HPV. Carbon monoxide (CO) is suggested as a positive regulator of Ca2+-activated K+ channel (BK(Ca)), a stimulator of guanylate cyclase, and an O2-mimetic agent in heme moiety-dependent O2 sensing mechanisms. Here we compared the effects of CO on the HPV (Po2, 3%) in isolated pulmonary artery (HPV(PA)) and in blood-perfused/ventilated lungs (HPV(lung)) of rats. A pretreatment with CO (3%) abolished the HPV(PA) in a reversible manner. The inhibition of HPV(PA) was completely reversed by 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ), a guanylate cyclase inhibitor. In contrast, the HPV(lung) was only partly decreased by CO. Moreover, the partial inhibition of HPV(lung) by CO was affected neither by the pretreatment with ODQ nor by NO synthase inhibitor (L-NAME). The CO-induced inhibitions of HPV(PA) and HPV(lung) were commonly unaffected by tetraethylammonium (TEA, 2 mM), a blocker of BK(Ca). As a whole, CO inhibits HPV(PA) via activating guanylate cyclase. The inconsistent effects of ODQ on HPV(PA) and HPV(lung) suggest that ODQ may lose its sGC inhibitory action when applied to the blood-containing perfusate.
Keyword
MeSH Terms
-
Animals
Anoxia/*physiopathology
Carbon Monoxide/*pharmacology
Guanylate Cyclase/antagonists & inhibitors/metabolism
NG-Nitroarginine Methyl Ester/chemistry/pharmacology
Nitric Oxide Synthase/antagonists & inhibitors/metabolism
Oxadiazoles/chemistry/pharmacology
Pulmonary Artery/*physiopathology
Quinoxalines/chemistry/pharmacology
Rats
Tetraethylammonium/chemistry/pharmacology
Vasoconstriction/*drug effects/physiology
Figure
Reference
-
1. Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gudermann T, Schulz R, Seeger W, Grimminger F, Weissmann N. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J. 2008. 32:1639–1651.
Article2. Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006. 570:53–58.
Article3. Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol. 2005. 98:415–420.
Article4. Duprat F, Lauritzen I, Patel A, Honoré E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007. 30:573–580.
Article5. Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, Wilhelm J, Morty RE, Brau ME, Weir EK, Kwapiszewska G, Klepetko W, Seeger W, Olschewski H. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res. 2006. 98:1072–1080.
Article6. Park SJ, Chun YS, Park KS, Kim SJ, Choi SO, Kim HL, Park JW. Identification of subdomains in NADPH oxidase-4 critical for the oxygen-dependent regulation of TASK-1 K+ channels. Am J Physiol Cell Physiol. 2009. 297:C855–C864.7. Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006. 10:672–686.
Article8. Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004. 26:270–280.
Article9. Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006. 203:2109–2119.
Article10. Ryter SW, Morse D, Choi AM. Carbon monoxide: to boldly go where NO has gone before. Sci STKE. 2004. 230:RE6.
Article11. Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004. 306:2093–2097.
Article12. Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005. 97:805–812.13. Vassalli F, Pierre S, Julien V, Bouckaert Y, Brimioulle S, Naeije R. Inhibition of hypoxic pulmonary vasoconstriction by carbon monoxide in dogs. Crit Care Med. 2001. 29:359–366.
Article14. Zhang F, Kaide JI, Yang L, Jiang H, Quan S, Kemp R, Gong W, Balazy M, Abraham NG, Nasjletti A. CO modulates pulmonary vascular response to acute hypoxia: relation to endothelin. Am J Physiol Heart Circ Physiol. 2004. 286:H137–H144.
Article15. Miller MA, Hales CA. Role of cytochrome P-450 in alveolar hypoxic pulmonary vasoconstriction in dogs. J Clin Invest. 1979. 64:666–673.
Article16. Tamayo L, Lopez-Lopez JR, Castaneda J, Gonzalez C. Carbon monoxide inhibits hypoxic pulmonary vasoconstriction in rats by a cGMP-independent mechanism. Pflugers Arch. 1997. 434:698–704.
Article17. Brunner F, Stessel H, Kukovetz WR. Novel guanylyl cyclase inhibitor, ODQ reveals role of nitric oxide, but not of cyclic GMP in endothelin-1 secretion. FEBS Lett. 1995. 376:262–266.
Article18. Mingone CJ, Gupte SA, Quan S, Abraham NG, Wolin MS. Influence of heme and heme oxygenase-1 transfection of pulmonary microvascular endothelium on oxidant generation and cGMP. Exp Biol Med (Maywood). 2003. 228:535–539.
Article19. Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2009. 47:93–107.
Article20. Baek EB, Yoo HY, Park SJ, Kim HS, Kim SD, Earm YE, Kim SJ. Luminal ATP-induced contraction of rabbit pulmonary arteries and role of purinoceptors in the regulation of pulmonary arterial pressure. Pflugers Arch. 2008. 457:281–291.
Article21. Sokal JA. Lack of the correlation between biochemical effects on rats and blood carboxyhemoglobin concentrations in various conditions of single acute exposure to carbon monoxide. Arch Toxicol. 1975. 34:331–336.
Article22. Lee YK, Kim EJ, Lee JE, Noh JW, Kim YG. Hypoxia induces connective tissue growth factor mRNA expression. J Korean Med Sci. 2009. 24:S176–S182.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulmonary edema in acute carbon monoxide poisoning
- Cerebral Infarction Followed by Hemorrhagic Transformation Accompanied by Carbon Monoxide Poisoning
- The effect of carbon monoxide on the [3H] 5-hydroxytryptamine binding sites in neonatal rats
- Induction of the c-fos in Rat Brain after Acute Carbon Monoxide Exposure
- A Clinical Observation of Acute Carbon Monoxide Poisoning