Ann Dermatol.
2015 Dec;27(6):791-792. 10.5021/ad.2015.27.6.791.
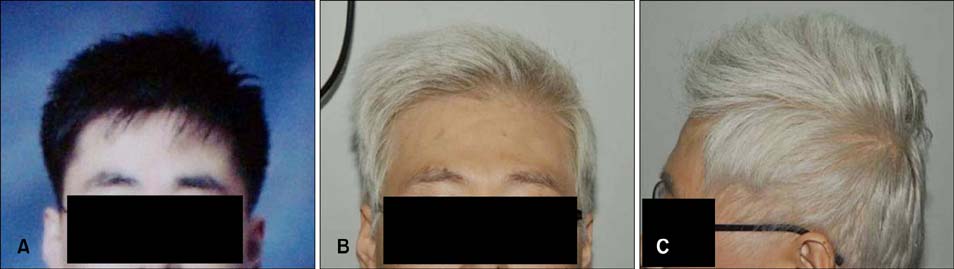
Gray Hair Associated with the Multitargeted Receptor Tyrosine Kinase Inhibitor Pazopanib
- Affiliations
-
- 1Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea. cymed@cnu.ac.kr
- 2Department of Dermatology, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 2157470
- DOI: http://doi.org/10.5021/ad.2015.27.6.791
Abstract
- No abstract available.
Figure
Reference
-
1. Routhouska S, Gilliam AC, Mirmirani P. Hair depigmentation during chemotherapy with a class III/V receptor tyrosine kinase inhibitor. Arch Dermatol. 2006; 142:1477–1479.
Article2. Moss KG, Toner GC, Cherrington JM, Mendel DB, Laird AD. Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther. 2003; 307:476–480.
Article3. Al-Marrawi MY, Rini B. Pazopanib for the treatment of renal cancer. Expert Opin Pharmacother. 2011; 12:1171–1189.
Article4. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013; 369:722–731.
Article5. Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010; 28:1061–1068.
Article6. Hartmann JT, Kanz L. Sunitinib and periodic hair depigmentation due to temporary c-KIT inhibition. Arch Dermatol. 2008; 144:1525–1526.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pazopanib for Non-small Cell Lung Cancer: The First Case Report in Korea
- A Case of Hair Change and Acneiform Eruption Induced by ZD1839 (Iressa(R))
- Treatment of a Patient with Kaposi's Sarcoma Arising during Hemodialysis with the Multikinase Inhibitor Pazopanib
- Erlotinib (Tarceva(R)) Induced Hair Abnormalities
- Management of Severe Fatigue Induced by Tyrosine Kinase Inhibitor in Radioiodine Refractory Thyroid Cancer