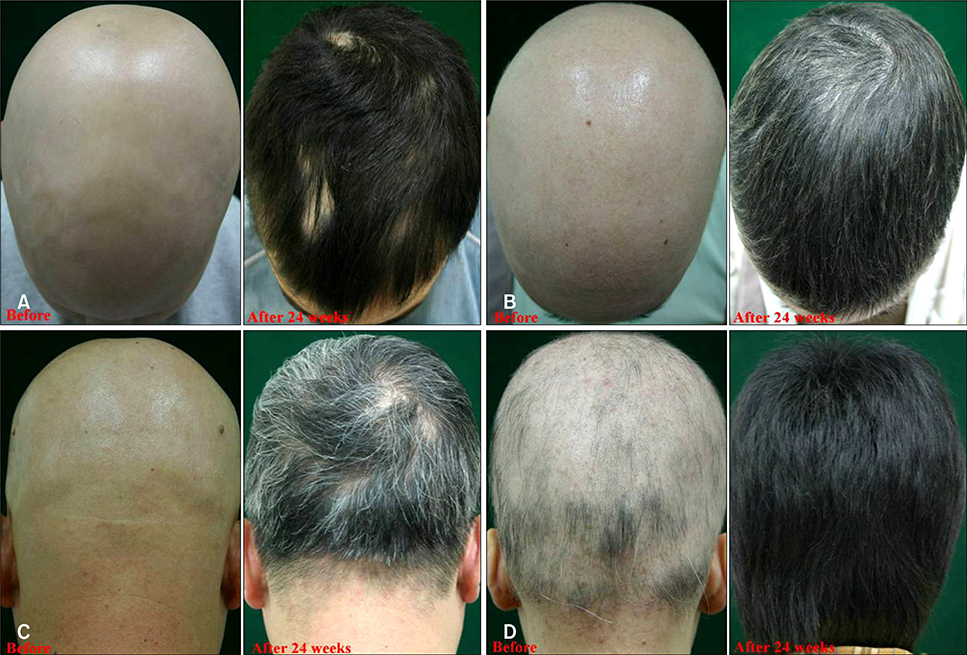
Ann Dermatol.
2008 Dec;20(4):172-178. 10.5021/ad.2008.20.4.172.
Treatment of Severe Alopecia Areata: Combination Therapy Using Systemic Cyclosporine A with Low Dose Corticosteroids
- Affiliations
-
- 1Department of Dermatology, Inje University School of Medicine, Busan, Korea. deany77@paran.com
- 2Gowoonsesang Dermatology Clinic, Busan, Korea.
- 3Clinical Trial Center, Busan Paik Hospital, Busan, Korea.
- KMID: 2156388
- DOI: http://doi.org/10.5021/ad.2008.20.4.172
Abstract
-
BACKGROUND: Combination therapy using cyclosporine A (CsA) together with low-dose corticosteroids has adequate efficacy with little toxicity for the treatment of severe alopecia areata (AA).
OBJECTIVE
We wanted to evaluate the clinical efficacy of combination therapy using CsA with low-dose corticosteroid for the treatment of severe AA and we also wanted to determine the safe therapeutic concentration of CsA in the peripheral blood.
METHODS
We treated 34 cases of severe AA with combination therapy for 24 weeks and we evaluated the efficacy at 12 and 24 weeks. We monitored the peripheral blood concentration of CsA to determine the therapeutic range of CsA that has the fewest side effects.
RESULTS
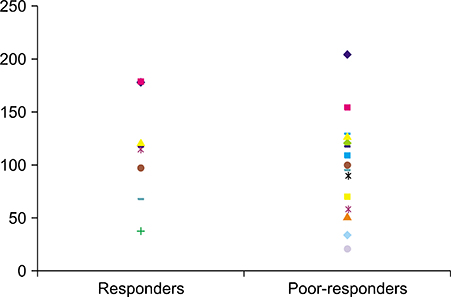
Of the patients, 77.4% (n=24) and 22.6% (n=10) were classified in the responder and poor-responder groups, respectively. The mean trough concentration of CsA was 95.1 and 101.2 ng/ml in the responder and poor-responder groups, respectively. For the patients with side effects associated with CsA, the mean CsA concentration was 195.8 ng/ml.
CONCLUSION
We found that combination therapy with systemic CsA and low-dose corticosteroids effectively treats severe AA and this therapy results in a safe, therapeutic concentration of CsA in the peripheral blood.
MeSH Terms
Figure
Reference
-
1. Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003; 49:96–98.
Article2. Feldmann KA, Kunte C, Wollenberg A, Wolfe H. Is topical tacrolimus effective in alopecia areata universalis? Br J Dermatol. 2002; 147:1031–1032.
Article3. Kurosawa M, Nakagawa S, Mizuashi M, Sasaki Y, Kawamura M, Saito M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology. 2006; 212:361–365.
Article4. Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005; 52:287–290.
Article5. Alabdulkareem AS, Abahussein AA, Okoro A. Severe alopecia areata treated with systemic corticosteroids. Int J Dermatol. 1998; 37:622–624.
Article6. Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998; 39:751–761.
Article7. Yoshizawa Y, Izaki S, Kitamura K, Kawana S. Systemic immunotherapy with topical dinitrochlorobenzene as additional treatment of alopecia areata. Acta Derm Venereol. 2002; 82:136–138.
Article8. Behrens-Williams SC, Leiter U, Schiener R, Weidmann M, Peter RU, Kerscher M. The PUVA-turban as a new option of applying a dilute psoralen solution selectively to the scalp of patients with alopecia areata. J Am Acad Dermatol. 2001; 44:248–252.
Article9. Oliver RF, Lowe JG. Oral cyclosporin A restores hair growth in the DEBR rat model for alopecia areata. Clin Exp Dermatol. 1995; 20:127–131.
Article10. Gafter-Gvili A, Sredni B, Gal R, Gafter U, Kalechman Y. Cyclosporin A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003; 284:C1593–C1603.
Article11. Ferron GM, Pyszczynski NA, Jusko WJ. Gender-related assessment of cyclosporine/prednisolone/sirolimus interactions in three human lymphocyte proliferation assays. Transplantation. 1998; 65:1203–1209.
Article12. Teshima H, Urabe A, Irie M, Nakagawa T, Nakayama J, Hori Y. Alopecia universalis treated with oral cyclosporine A and prednisolone: immunologic studies. Int J Dermatol. 1992; 31:513–516.
Article13. Shapiro J, Lui H, Tron V, Ho V. Systemic cyclosporine and low-dose prednisone in the treatment of chronic severe alopecia areata: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1997; 36:114–117.
Article14. Park HH, Sim WY. Cyclosporine combination therapy in alopecia areata. Korean J Dermatol. 2002; 40:1311–1315.15. Umezawa Y, Ozawa A. Optimal time for therapeutic drug monitoring of cyclosporine microemulsion in patients with psoriasis. Int J Dermatol. 2007; 46:763–766.
Article16. Wang CH, Chou NK, Wu FL, Ko WJ, Tsao CI, Chi NH, et al. Therapeutic drug monitoring of cyclosporine Neoral in de novo Chinese cardiac transplant recipients treated with an everolimus-cyclosporine immunosuppressive regimen. Transplant Proc. 2006; 38:2132–2134.
Article17. Rinaldi S, Sesto A, Barsotti P, Faraggiana T, Sera F, Rizzoni G. Cyclosporine therapy monitored with abbreviated area under curve in nephrotic syndrome. Pediatr Nephrol. 2005; 20:25–29.
Article18. Ferrando J, Grimalt R. Partial response of severe alopecia areata to cyclosporine A. Dermatology. 1999; 199:67–69.
Article19. Gafter-Gvili A, Kalechman Y, Sredni B, Gal R, Gafter U. Cyclosporin A-induced hair growth in mice is associated with inhibition of hair follicle regression. Arch Dermatol Res. 2004; 296:265–269.
Article20. Grevel J. Area-under-the-curve versus trough level monitoring of cyclosporine concentration: critical assessment of dosage adjustment practices and measurement of clinical outcome. Ther Drug Monit. 1993; 15:488–491.
Article21. Armstrong VW, Oellerich M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin Biochem. 2001; 34:9–16.
Article22. Kim BJ, Min SU, Park KY, Choi JW, Park SW, Youn SW, et al. Combination therapy of cyclosporine and methylprednisolone on severe alopecia areata. J Dermatolog Treat. 2008; 19:216–220.
Article23. Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006; 112:184–198.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Alopecia Areata in a Patient taking Cyclosporine
- Comparison of Therapeutic Effect of High Dose Corticosteroid Pulse Therapy and Combination Therapy of Cyclosporine with Low Does Corticosteroid for Severe Alopecia Areata
- Comparison of High-Dose Corticosteroid Pulse Therapy and Combination Therapy Using Oral Cyclosporine with Low-Dose Corticosteroid in Severe Alopecia Areata
- Cyclosporine Combination Therapy in Alopecia Areata
- Effect of Combined Therapy of Systemic Corticosteroid with Topical Agents in Treatment of Severe Alopecia Areata