J Vet Sci.
2014 Sep;15(3):423-426. 10.4142/jvs.2014.15.3.423.
A reverse transcription loop-mediated isothermal amplification assay to rapidly diagnose foot-and-mouth disease virus C
- Affiliations
-
- 1State Key Laboratory of Veterinary Etiological Biology, OIE/National Foot and Mouth Disease Reference Laboratory, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou 730046, China. zhangjie03@caas.cn
- 2Jiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, Jiangsu 225009, China.
- 3Lanzhou Jiaotong Univesity Bowen College, Lanzhou 730101, China.
- KMID: 2155626
- DOI: http://doi.org/10.4142/jvs.2014.15.3.423
Abstract
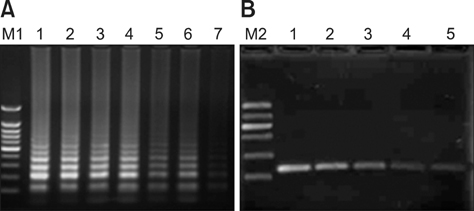
- A reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay was developed to rapidly detect foot-and-mouth disease virus serotype C (FMDV C). By testing 10-fold serial dilutions of FMDV C samples, sensitivity of the FMDV C RT-LAMP was found to be 10 times higher than that of conventional reverse transcription-PCR (RT-PCR). No cross-reactivity with A, Asia 1, or O FMDV or swine vesicular disease virus (SVDV) indicated that FMDV C RT-LAMP may be an exciting novel method for detecting FMDV C.
Keyword
MeSH Terms
Figure
Reference
-
1. Ayebazibwe C, Mwiine FN, Balinda SN, Tjørnehøj K, Masembe C, Muwanika VB, Okurut ARA, Siegismund HR, Alexandersen S. Antibodies against foot-and-mouth disease (FMD) virus in African buffalos (Syncerus caffer) in selected National Parks in Uganda (2001-2003). Transbound Emerg Dis. 2010; 57:286–292.
Article2. Carrillo C, Tulman ER, Delhon G, Lu Z, Carreno A, Vagnozzi A, Kutish GF, Rock DL. Comparative genomics of foot-and-mouth disease virus. J Virol. 2005; 79:6487–6504.
Article3. Farag MA, Halima EW, Abeer AT. Detection of FMD Virus using A Dot Immunoblot and RT-PCR from field Samples. 1rst Ann Confr FVM Moshtohor. 2004. p. 67–77.4. Fernández J, Agüero M, Romero L, Sánchez C, Belák S, Arias M, Sánchez-Vizcaíno JM. Rapid and differential diagnosis of foot-and-mouth disease, swine vesicular disease, and vesicular stomatitis by a new multiplex RT-PCR assay. J Virol Methods. 2008; 147:301–311.
Article5. Food and Agriculture Organization of the United Nations. Foot-and-mouth disease: situation worldwide and major epidemiological events in 2005-2006. FAO EMPRES. 2007; 1:1–11.6. Grubman MJ, Baxt B. Foot-and-mouth disease. Clin Microbiol Rev. 2004; 17:465–493.
Article7. Knowles NJ, Samuel AR. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003; 91:65–80.
Article8. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000; 28:E63.
Article9. Paton DJ, Sumption KJ, Charleston B. Options for control of foot-and-mouth disease: knowledge, capability and policy. Philos Trans R Soc Lond B Biol Sci. 2009; 364:2657–2667.
Article10. Sangula AK, Siegismund HR, Belsham GJ, Balinda SN, Masembe C, Muwanika VB. Low diversity of foot-and-mouth disease serotype C virus in Kenya: evidence for probable vaccine strain re-introductions in the field. Epidemiol Infect. 2011; 139:189–196.
Article11. Tekleghiorghis T, Moormann RJM, Weerdmeester K, Dekker A. Serological Evidence Indicates that Foot-and- Mouth Disease Virus Serotype O, C and SAT1 are most Dominant in Eritrea. Transbound Emerg Dis. 2013; Epub ahead of print. doi: 10.1111/tbed.12065.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uracil-DNA glycosylase-treated reverse transcription loop-mediated isothermal amplification for rapid detection of avian influenza virus preventing carry-over contamination
- Loop-mediated isothermal amplification assay for the detection of Salmonella spp. in pig feces
- Evaluation of a Direct Reverse Transcription Loop-Mediated Isothermal Amplification Method without RNA Extraction (Direct RT-LAMP) for the Detection of Lymph Node Metastasis in Early Breast Cancer
- Development of reverse-transcription loop-mediated isothermal amplification assays for point-of-care testing of human influenza virus subtypes H1N1 and H3N2
- Development of reverse transcription loop-mediated isothermal amplification assays for point-of-care testing of avian influenza virus subtype H5 and H9