J Vet Sci.
2014 Sep;15(3):361-367. 10.4142/jvs.2014.15.3.361.
Detection of porcine reproductive and respiratory syndrome virus in oral fluid from naturally infected pigs in a breeding herd
- Affiliations
-
- 1Department of Veterinary Pathology, Faculty of Agriculture, University of Miyazaki, Miyazaki 889-2192, Japan. t-hirai@cc.miyazaki-u.ac.jp
- 2Department of Veterinary Public Health, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Hanoi, Vietnam.
- 3Department of Veterinary Pathology, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Hanoi, Vietnam.
- KMID: 2155619
- DOI: http://doi.org/10.4142/jvs.2014.15.3.361
Abstract
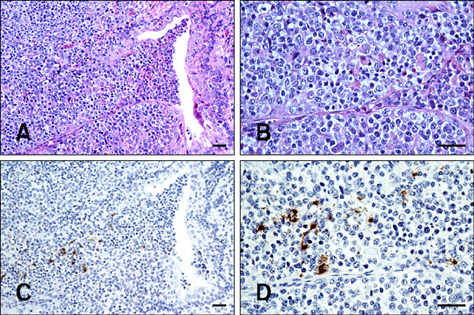
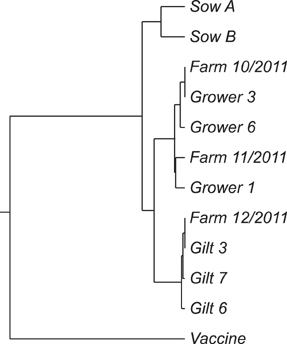
- The objectives of the present study were to evaluate the anatomic localization of porcine reproductive and respiratory syndrome virus (PRRSV) in naturally infected pigs and to determine whether oral fluid could be used to detect the virus in infected animals. Two sows, seven 2-month-old grower pigs, and 70 6-month-old gilts were included in this study. PRRSV in sera and oral fluid were identified by nested reverse transcription PCR (nRT-PCR) while lung, tonsil, and tissue associated with oral cavity were subjected to nRT-PCR, immunohistochemistry, and in situ hybridization. In sows, PRRSV was identified in oral fluid and tonsils. PRRSV was also detected in oral fluid, tonsils, salivary glands, oral mucosa, and lungs of all seven grower pigs. However, viremia was observed in only two grower pigs. Double staining revealed that PRRSV was distributed in macrophages within and adjacent to the tonsillar crypt epithelium. In gilts, the North American type PRRSV field strain was detected 3 to 8 weeks after introducing these animals onto the farm. These results confirm previous findings that PRRSV primarily replicates in tonsils and is then shed into oral fluid. Therefore, oral fluid sampling may be effective for the surveillance of PRRSV in breeding herds.
Keyword
MeSH Terms
-
Animals
Female
In Situ Hybridization/veterinary
Lung/virology
Male
Palatine Tonsil/virology
Polymerase Chain Reaction/veterinary
Porcine Reproductive and Respiratory Syndrome/*virology
Porcine respiratory and reproductive syndrome virus/*physiology
Saliva/*virology
Salivary Glands/virology
Swine/virology
Virus Replication/physiology
Figure
Reference
-
1. Andreyev VG, Wesley RD, Mengeling WL, Vorwald AC, Lager KM. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch Virol. 1997; 142:993–1001.
Article2. Benson JE, Yaeger MJ, Christopher-Hennings J, Lager K, Yoon KJ. A comparison of virus isolation, immunohistochemistry, fetal serology, and reverse-transcription polymerase chain reaction assay for the identification of porcine reproductive and respiratory syndrome virus transplacental infection in the fetus. J Vet Diagn Invest. 2002; 14:8–14.
Article3. Bierk MD, Dee SA, Rossow KD, Collins JE, Guedes MI, Molitor TW. Experiences with tonsil biopsy as an antemortem diagnostic test for detecting porcine reproductive and respiratory syndrome virus infection in breeding swine. Swine Health Prod. 2000; 8:279–282.4. Bierk MD, Dee SA, Rossow KD, Collins JE, Guedes MI, Pijoan C, Molitor TW. Diagnostic investigation of chronic porcine reproductive and respiratory syndrome virus in a breeding herd of pigs. Vet Rec. 2001; 148:687–690.
Article5. Choi C, Chae C. Colocalization of porcine reproductive and respiratory syndrome virus and porcine circovirus 2 in porcine dermatitis and nephrology syndrome by double-labeling technique. Vet Pathol. 2001; 38:436–441.
Article6. Cheon DS, Chae C. Comparison of virus isolation, reverse transcription-polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine reproductive and respiratory syndrome virus from naturally aborted fetuses and stillborn piglets. J Vet Diagn Invest. 2000; 12:582–587.
Article7. Cheon DS, Chae C. Distribution of porcine reproductive and respiratory syndrome virus in stillborn and liveborn piglets from experimentally infected sows. J Comp Pathol. 2001; 124:231–237.
Article8. Christianson WT, Collins JE, Benfield DA, Harris L, Gorcyca DE, Chladek DW, Morrison RB, Joo HS. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am J Vet Res. 1992; 53:485–488.9. Christopher-Hennings J, Holler LD, Benfield DA, Nelson EA. Detection and duration of porcine reproductive and respiratory syndrome virus in semen, serum, peripheral blood mononuclear cells, and tissues from Yorkshire, Hampshire, and Landrace boars. J Vet Diagn Invest. 2001; 13:133–142.
Article10. Fano E, Olea L, Pijoan C. Eradication of porcine reproductive and respiratory syndrome virus by serum inoculation of naïve gilts. Can J Vet Res. 2005; 69:71–74.11. Karniychuk UU, Saha D, Geldhof M, Vanhee M, Cornillie P, Van den Broeck W, Nauwynck HJ. Porcine reproductive and respiratory syndrome virus (PRRSV) causes apoptosis during its replication in fetal implantation sites. Microb Pathog. 2011; 51:194–202.
Article12. Kittawornrat A, Prickett J, Chittick W, Wang C, Engle M, Johnson J, Patnayak D, Schwartz T, Whitney D, Olsen C, Schwartz K, Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010; 154:170–176.
Article13. Ogawa H, Taira O, Hirai T, Takeuchi H, Nagao A, Ishikawa Y, Tuchiya K, Nunoya T, Ueda S. Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J Virol Methods. 2009; 160:210–214.
Article14. Pepin BJ, Kittawornrat A, Liu F, Gauger PC, Harmon K, Abate S, Main R, Garton C, Hargrove J, Rademacher C, Ramirez A, Zimmerman J. Comparison of specimens for detection of Porcine reproductive and respiratory syndrome virus infection in boar studs. Transbound Emerg Dis. 2013; Epub ahead of print. doi:10.1111/tbed.12135.
Article15. Prickett JR, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008; 20:156–163.
Article16. Rossow KD. Porcine reproductive and respiratory syndrome. Vet Pathol. 1998; 35:1–20.
Article17. Rowland RRR, Lawson S, Rossow K, Benfield DA. Lymphoid tissue tropism of porcine reproductive and respiratory syndrome virus replication during persistent infection of pigs originally exposed to virus in utero. Vet Microbiol. 2003; 96:219–235.
Article18. Scortti M, Prieto C, Simarro I, Castro JM. Reproductive performance of gilts following vaccination and subsequent heterologous challenge with European strains of porcine reproductive and respiratory syndrome virus. Theriogenology. 2006; 66:1884–1893.
Article19. Tanizaki Y, Sato Y, Oka H, Utsuki S, Kondo K, Miyajima Y, Nagashio R, Fujii K. Expression of autocrine motility factor mRNA is a poor prognostic factor in high-grade astrocytoma. Pathol Int. 2006; 56:510–515.
Article20. Vashisht K, Erlandson KR, Firkins LD, Zuckermann FA, Goldberg TL. Evaluation of contact exposure as a method for acclimatizing growing pigs to porcine reproductive and respiratory syndrome virus. J Am Vet Med Assoc. 2008; 232:1530–1535.
Article21. Wills RW, Zimmerman JJ, Yoon KJ, Swenson SL, Hoffman LJ, McGinley MJ, Hill HT, Platt KB. Porcine reproductive and respiratory syndrome virus: routes of excretion. Vet Microbiol. 1997; 57:69–81.
Article22. Yoshii M, Kaku Y, Murakami Y, Shimizu M, Kato K, Ikeda H. Polymerase chain reaction-based genetic typing of Japanese porcine reproductive and respiratory syndrome viruses. J Vet Diagn Invest. 2004; 16:342–347.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic studies in lymph nodes of pigs infected with porcine circovirus type 2, porcine reproductive and respiratory syndrome virus
- Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology
- Prevalence of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2 and porcine parvovirus from aborted fetuses and pigs with respiratory problems in Korea
- Acute porcine reproductive and respiratory syndrome outbreaksin immunized sow herds: from occurrence to stabilization under whole herd vaccination strategy
- Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method