J Vet Sci.
2014 Sep;15(3):353-359. 10.4142/jvs.2014.15.3.353.
Kinetin inhibits apoptosis of aging spleen cells induced by D-galactose in rats
- Affiliations
-
- 1College of Veterinary Medicine, Northwest A&F University, Yangling 712100, China. oywq2014@163.com
- 2College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471003, China.
- KMID: 2155618
- DOI: http://doi.org/10.4142/jvs.2014.15.3.353
Abstract
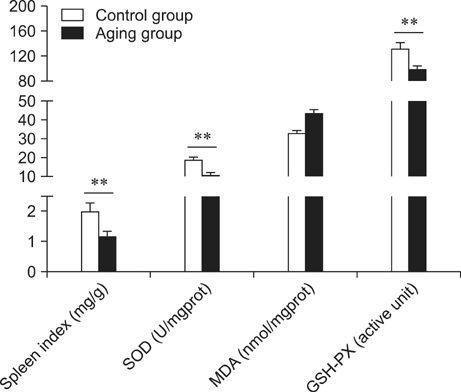
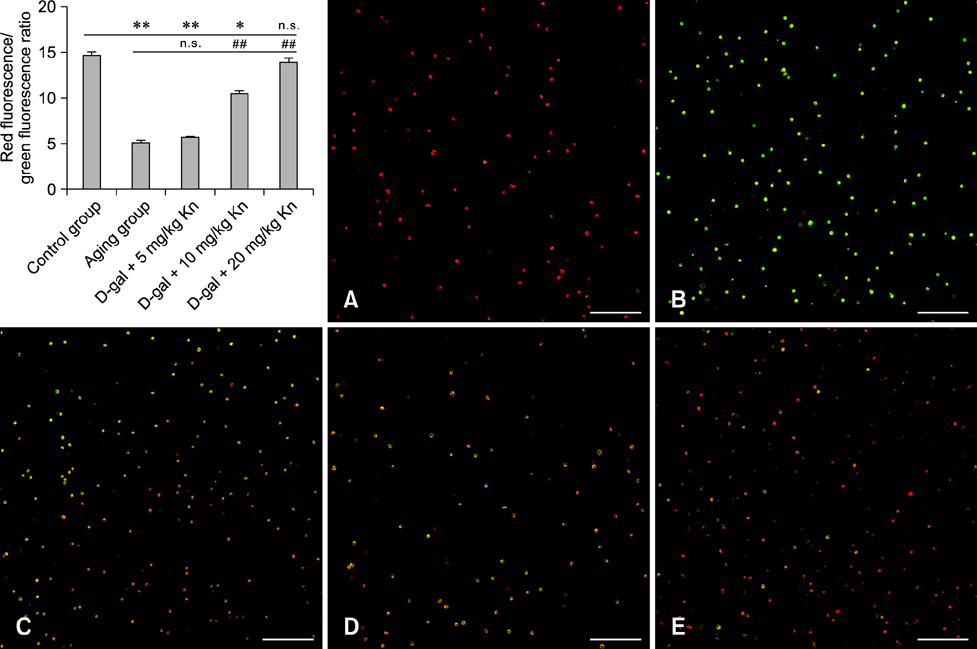
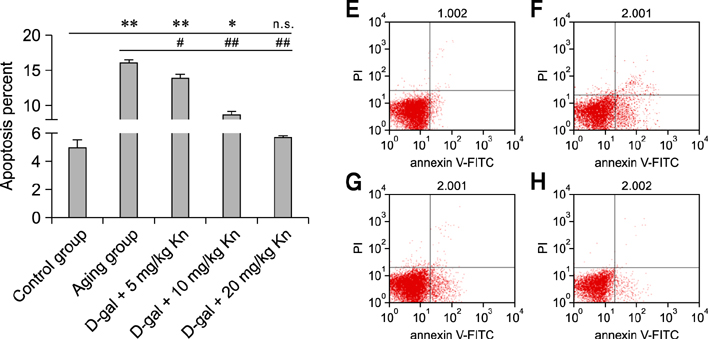
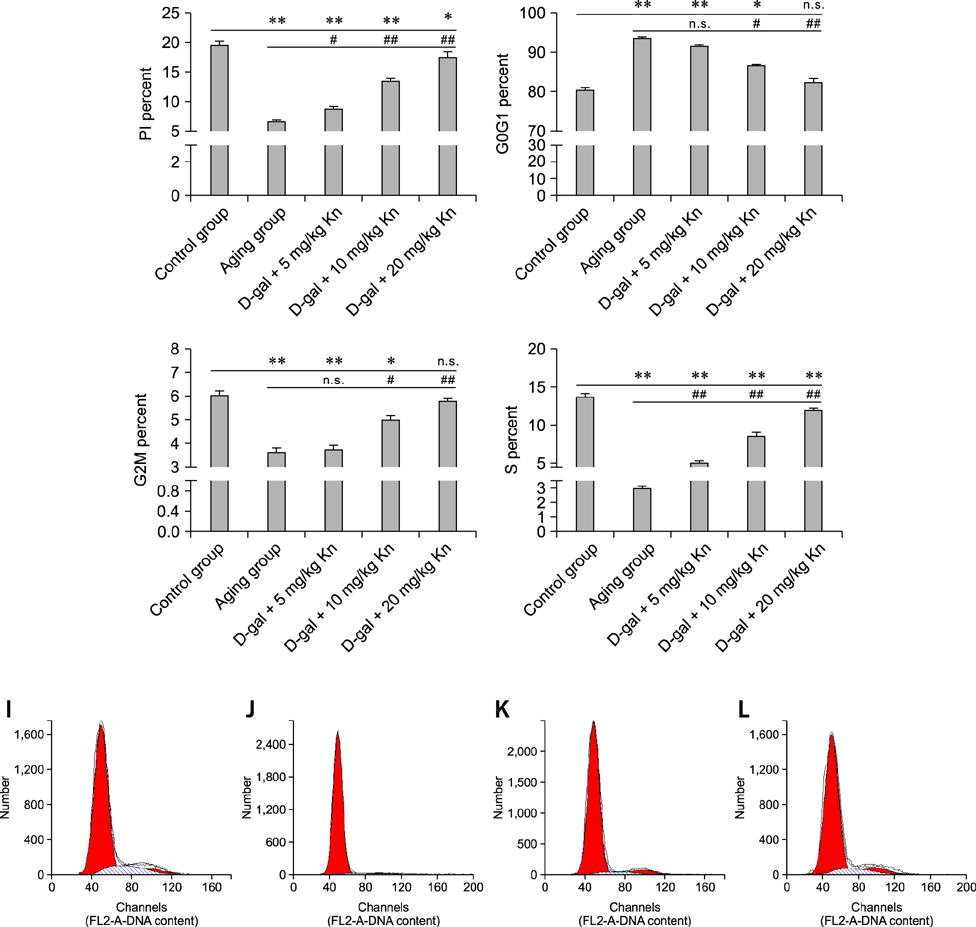
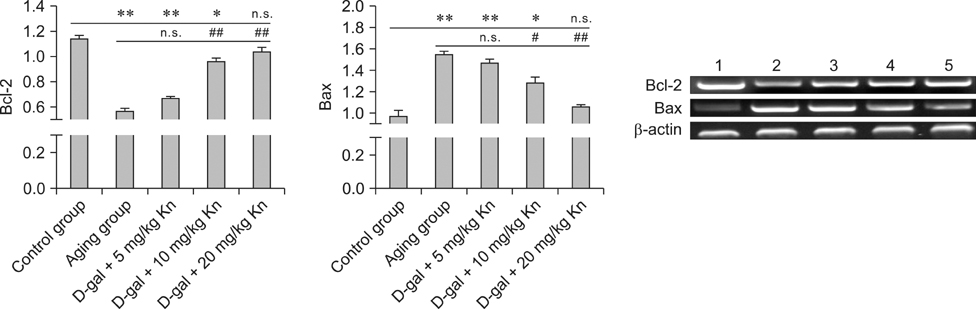
- Kinetin (Kn) is a cytokinin growth factor that exerts several anti-aging and antioxidant effects on cells and organs. To investigate the mechanism underlying apoptotic events in aging cells induced by D-galactose (D-gal), we examined the effect of Kn delivered via nuchal subcutaneous injection on D-gal-induced aging and apoptosis in rats. Our results showed that interleukin (IL)-2 levels and mitochondrial membrane potential (DeltaPsim) were decreased by Kn in aging rats while IL-6 production and apoptosis increased. In addition, the expression of anti-apoptotic Bcl-2 was low while that of Bax was high in the aging group. After treated with Kn, compared with aging group, there showed obvious difference in Kn group with elevated IL-2, proliferation index, Bcl-2, DeltaPsim and decreased IL-6 and Bax in splenic lymphocyte. Based on these results, we concluded that Kn can effectively protect the rat spleen from aging, apoptosis, and atrophy.
Keyword
MeSH Terms
-
Aging/drug effects/physiology
Animals
Apoptosis/drug effects/*physiology
Female
Galactose/*pharmacology
Interleukin-6/physiology
Interleukins/physiology
Kinetin/pharmacology/*physiology
Male
Membrane Potential, Mitochondrial/drug effects/physiology
Rats
Spleen/*cytology/drug effects/physiology
Galactose
Interleukin-6
Interleukins
Kinetin
Figure
Reference
-
1. Alemi M, Sabouni F, Sanjarian F, Haghbeen K, Ansari S. Anti-inflammatory effect of seeds and callus of Nigella sativa L. extracts on mix glial cells with regard to their thymoquinone content. AAPS PharmSciTech. 2013; 14:160–167.
Article2. Aw D, Silva AB, Palmer DB. Is thymocyte development functional in the aged? Aging (Albany NY). 2009; 1:146–153.
Article3. Basta-Kaim A, Szczęsny E, Leśkiewicz M, Głombik K, Slusarczyk J, Budziszewska B, Regulska M, Kubera M, Nowak W, Wędzony K, Lasoń W. Maternal immune activation leads to age-related behavioral and immunological changes in male rat offspring - the effect of antipsychotic drugs. Pharmacol Rep. 2012; 64:1400–1410.
Article4. Boone N, Loriod B, Bergon A, Sbai O, Formisano-Tréziny C, Gabert J, Khrestchatisky M, Nguyen C, Féron F, Axelrod FB, Ibrahim EC. Olfactory stem cells, a new cellular model for studying molecular mechanisms underlying familial dysautonomia. PLoS One. 2010; 5:e15590.
Article5. Chiu PC, Chan CC, Lin HM, Chiu HC. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: a randomized, double-blind, placebo-controlled, split-face comparative trial. J Cosmet Dermatol. 2007; 6:243–249.
Article6. Dkhar P, Sharma R. Late-onset dietary restriction modulates protein carbonylation and catalase in cerebral hemispheres of aged mice. Cell Mol Neurobiol. 2014; 34:307–313.
Article7. Hošek J, Novotná R, Babula P, Vančo J, Trávníček Z. Zn(II)-chlorido complexes of phytohormone kinetin and its derivatives modulate expression of inflammatory mediators in THP-1 cells. PLoS One. 2013; 8:e65214.
Article8. Kumar D, Rizvi SI. Plasma paraoxonase 1 arylesterase activity in D-galactose-induced aged rat model: correlation with LDL oxidation and redox status. Aging Clin Exp Res. 2014; 26:261–267.
Article9. Lin HP, Lin CY, Hsiao PH, Wang HD, Sheng Jiang S, Hsu JM, Jim WT, Chen M, Kung HJ, Chuu CP. Difference in protein expression profile and chemotherapy drugs response of different progression stages of LNCaP sublines and other human prostate cancer cells. PLoS One. 2013; 8:e82625.
Article10. Martin-Cano FE, Camello-Almaraz C, Acuña-Castroviejo D, Pozo MJ, Camello PJ. Age-related changes in mitochondrial function of mouse colonic smooth muscle: beneficial effects of melatonin. J Pineal Res. 2013; Epub ahead of print. doi:10.1111/jpi.12109.
Article11. Meneguello-Coutinho M, Caperuto E, Bacurau AV, Chamusca G, Uchida MC, Tibana RA, Pereira GB, Navalta JW, Wasinski F, Cavaglieri CR, Prestes J, Costa Rosa LF, Bacurau RF. Effects of dietary restriction or swimming on lymphocytes and macrophages functionality from old rats. Immunol Invest. 2014; 43:113–122.
Article12. Mik V, Szüčová L, Smehilová M, Zatloukal M, Doležal K, Nisler J, Grúz J, Galuszka P, Strnad M, Spíchal L. N9-substituted derivatives of kinetin: effective antisenescence agents. Phytochemistry. 2011; 72:821–831.
Article13. Minutoli L, Bitto A, Squadrito F, Marini H, Irrera N, Morgia G, Passantino A, Altavilla D. Serenoa Repens, lycopene and selenium: a triple therapeutic approach to manage benign prostatic hyperplasia. Curr Med Chem. 2013; 20:1306–1312.
Article14. Mukherji R, Prabhune A. Novel glycolipids synthesized using plant essential oils and their application in quorum sensing inhibition and as antibiofilm agents. ScientificWorldJournal. 2014; 2014:890709.
Article15. Philippe L, Tosca L, Zhang WL, Piquemal M, Ciapa B. Different routes lead to apoptosis in unfertilized sea urchin eggs. Apoptosis. 2014; 19:436–450.
Article16. Rice KM, Meduru S, Kakarla SK, Katta A, Mupparaju SP, Kidd B, Goebel LJ, Blough ER. Chronic paracetamol treatment influences indices of reactive oxygen species accumulation in the aging Fischer 344 X Brown Norway rat aorta. Ann Clin Lab Sci. 2012; 42:152–161.17. Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer's disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008; 28:3051–3059.
Article18. Schöneich C, Dremina E, Galeva N, Sharov V. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis. 2014; 19:42–57.
Article19. Song S, Gao P, Xiao H, Xu Y, Si LY. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013; 8:e82968.
Article20. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997; 275:1129–1132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- L-histidine and L-carnosine exert anti-brain aging effects in D-galactose-induced aged neuronal cells
- Induction of apoptosis in mouse spleen cells by Ginsenoside Rp1
- Kinetin Improves Barrier Function of the Skin by Modulating Keratinocyte Differentiation Markers
- Esculetin, a Coumarin Derivative, Inhibits Aldose Reductase Activity in vitro and Cataractogenesis in Galactose-Fed Rats
- Curcumin and hesperetin attenuate D-galactose-induced brain senescence in vitro and in vivo