J Vet Sci.
2014 Sep;15(3):343-352. 10.4142/jvs.2014.15.3.343.
Antepartal insulin-like growth factor concentrations indicating differences in the metabolic adaptive capacity of dairy cows
- Affiliations
-
- 1Clinic for Cattle, University of Veterinary Medicine, 30173 Hannover, Germany. marion.piechotta@tiho-hannover.de
- 2Department of Animal Biology, Faculty of Veterinary Science, University of Zulia, Maracaibo, Zulia 44011, Venezuela.
- 3Department of Population Medicine and Diagnostic Sciences, College of Veterinary Medicine, Cornell University, Ithaca NY 14853, USA.
- 4Anatomy Unit, University of Veterinary Medicine, 30173 Hannover, Germany.
- 5Immunology Unit, University of Veterinary Medicine, 30173 Hannover, Germany.
- 6Clinic of Reproductive Medicine, Vetsuisse Faculty, University of Zurich, 8057 Zurich, Switzerland.
- KMID: 2155617
- DOI: http://doi.org/10.4142/jvs.2014.15.3.343
Abstract
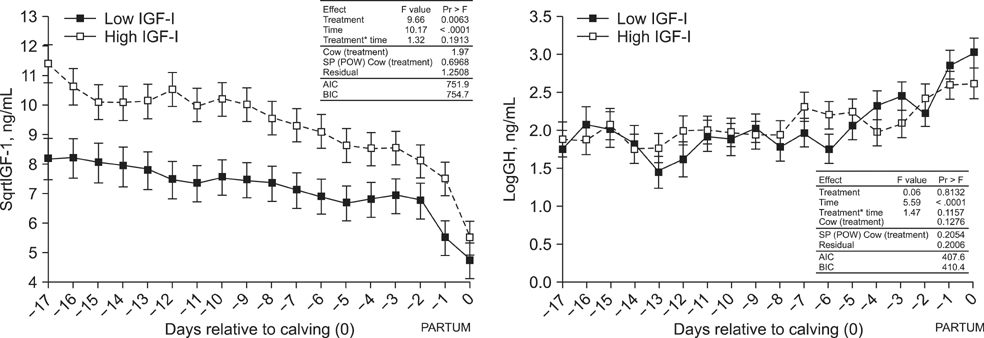
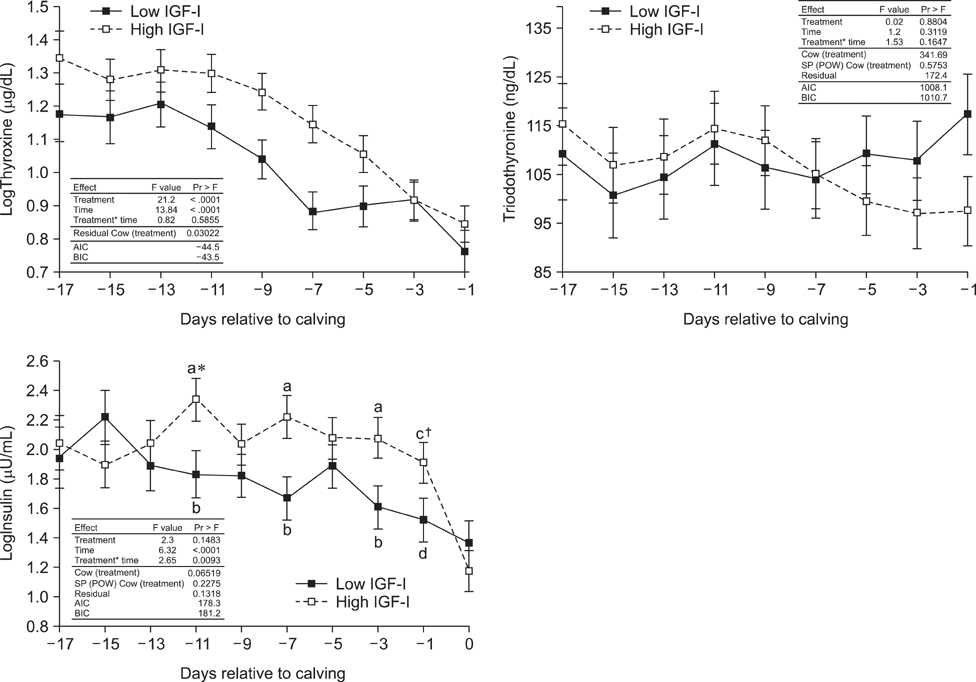
- Cows with different Insulin-like Growth Factor-I (IGF-I) concentrations showed comparable expression levels of hepatic growth hormone receptor (GHR). Suppressor of cytokine signaling 2 (SOCS2), could be responsible for additional inhibition of the GHR signal cascade. The aims were to monitor cows with high or low antepartal IGF-I concentrations (IGF-I(high) or IGF-I(low)), evaluate the interrelationships of endocrine endpoints, and measure hepatic SOCS2 expression. Dairy cows (n = 20) were selected (240 to 254 days after artificial insemination (AI)). Blood samples were drawn daily (day -17 until calving) and IGF-I, GH, insulin, thyroid hormones, estradiol, and progesterone concentrations were measured. Liver biopsies were taken (day 264 +/- 1 after AI and postpartum) to measure mRNA expression (IGF-I, IGFBP-2, IGFBP-3, IGFBP-4, acid labile subunit (ALS), SOCS2, deiodinase1, GHR1A). IGF-I concentrations in the two groups were different (p < 0.0001). However, GH concentrations and GHR1A mRNA expression were comparable (p > 0.05). Thyroxine levels and ALS expression were higher in the IGF-I(high) cows compared to IGF-I(low) cows. Estradiol concentration tended to be greater in the IGF-I(low) group (p = 0.06). It was hypothesized that low IGF-I levels are associated with enhanced SOCS2 expression although this could not be decisively confirmed by the present study.
Keyword
MeSH Terms
-
Animals
Cattle
Estradiol/blood
Female
Growth Hormone/blood
Insulin/blood
Insulin-Like Growth Factor Binding Protein 2/analysis
Insulin-Like Growth Factor Binding Protein 3/analysis
Insulin-Like Growth Factor Binding Protein 4/analysis
Insulin-Like Growth Factor I/*analysis/physiology
Liver/chemistry
Pregnancy/metabolism/physiology
Pregnancy, Animal/*metabolism/physiology
Progesterone/blood
Suppressor of Cytokine Signaling Proteins/analysis
Thyroid Hormones/blood
Estradiol
Growth Hormone
Insulin
Insulin-Like Growth Factor Binding Protein 2
Insulin-Like Growth Factor Binding Protein 3
Insulin-Like Growth Factor Binding Protein 4
Insulin-Like Growth Factor I
Progesterone
Suppressor of Cytokine Signaling Proteins
Thyroid Hormones
Figure
Cited by 1 articles
-
Serum IGFBP4 concentration decreased in dairy heifers towards day 18 of pregnancy
Marie M Meyerholz, Kirsten Mense, Michael Lietzau, Ana Kassens, Matthias Linden, Hendrike Knaack, Elisa Wirthgen, Andreas Hoeflich, Mariam Raliou, Christophe Richard, Olivier Sandra, Hans-Joachim Schuberth, Martina Hoedemaker, Marion Schmicke
J Vet Sci. 2015;16(4):413-421. doi: 10.4142/jvs.2015.16.4.413.
Reference
-
1. Aceves C, Ruiz A, Romero C, Valverde C. Homeorhesis during early lactation. Euthyroid sick-like syndrome in lactating cows. Acta Endocrinol (Copenh). 1985; 110:505–509.
Article2. Arbuckle JL. AMOS 18 User's Guide. Crawfordville: Amos Development Corporation;2009.3. Arbuckle JL. IBM SPSS Amos 19 User's Guide. Crawfordville: Amos Development Corporation;2010.4. Bagozzi RP, Yi Y. Specification, evaluation, and interpretation of structural equation models. J Acad Mark Sci. 2012; 40:8–34.
Article5. Bell AW. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci. 1995; 73:2804–2819.
Article6. Bradford BJ, Allen MS. Negative energy balance increases periprandial ghrelin and growth hormone concentrations in lactating dairy cows. Domest Anim Endocrinol. 2008; 34:196–203.
Article7. Clemmons DR, Underwood LE. Nutritional regulation of IGF-I and IGF binding proteins. Annu Rev Nutr. 1991; 11:393–412.
Article8. Hannon K, Trenkle A. Relationship of thyroid status to growth hormone and insulin-like growth factor-I (IGF-I) in plasma and IGF-I mRNA in liver and skeletal muscle of cattle. Domest Anim Endocrinol. 1991; 8:595–600.
Article9. Kam GY, Leung KC, Baxter RC, Ho KKY. Estrogens exert route- and dose-dependent effects on insulin-like growth factor (IGF)-binding protein-3 and the acid-labile subunit of the IGF ternary complex. J Clin Endocrinol Metab. 2000; 85:1918–1922.
Article10. Kawashima C, Sakaguchi M, Suzuki T, Sasamoto Y, Takahashi Y, Matsui M, Miyamoto A. Metabolic profiles in ovulatory and anovulatory primiparous dairy cows during the first follicular wave postpartum. J Reprod Dev. 2007; 53:113–120.
Article11. Kessel S, Stroehl M, Meyer HH, Hiss S, Sauerwein H, Schwarz FJ, Bruckmaier RM. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J Anim Sci. 2008; 86:2903–2912.
Article12. Kobayashi Y, Boyd CK, McCormack BL, Lucy MC. Reduced insulin-like growth factor-I after acute feed restriction in lactating dairy cows is independent of changes in growth hormone receptor 1A mRNA. J Dairy Sci. 2002; 85:748–754.
Article13. Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001; 22:53–74.
Article14. Leong GM, Moverare S, Brce J, Doyle N, Sjögren K, Dahlman-Wright K, Gustafsson JÅ, Ho KKY, Ohlsson C, Leung KC. Estrogen up-regulates hepatic expression of suppressors of cytokine signaling-2 and -3 in vivo and in vitro. Endocrinology. 2004; 145:5525–5531.
Article15. Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CKW, Low TH, Leong GM, Ross RJM, Ho KKY. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci U S A. 2003; 100:1016–1021.
Article16. Littell RC. Analysis of unbalanced mixed model data: a case study comparison of ANOVA versus REML/GLS. J Agric Biol Environ Stat. 2002; 7:472–490.
Article17. Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998; 76:1216–1231.
Article18. Littell RC, Milliken GA, Stroup WS, Wolfinger RD, Schabenberger O. SAS for Mixed Model. 2nd ed. Cary: SAS Institute;2006.19. Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000; 19:1793–1819.
Article20. Lucy MC, Jiang H, Kobayashi Y. Changes in the somatotrophic axis associated with the initiation of lactation. J Dairy Sci. 2001; 84:Suppl. E113–E119.
Article21. McGuire MA, Vicini JL, Bauman DE, Veenhuizen JJ. Insulin-like growth factors and binding proteins in ruminants and their nutritional regulation. J Anim Sci. 1992; 70:2901–2910.
Article22. Meikle A, Kulcsar M, Chilliard Y, Febel H, Delavaud C, Cavestany D, Chilibroste P. Effects of parity and body condition at parturition on endocrine and reproductive parameters of the cow. Reproduction. 2004; 127:727–737.
Article23. Nowak W, Mikula R, Pruszyńska-Oszmalek E, Maćkowiak P, Stefańska B, Kasprowicz-Potocka M, Frankiewicz A, Drzazga K. Dietary energy density in the dry period on the metabolic status of lactating cows. Pol J Vet Sci. 2013; 16:715–722.
Article24. Piechotta M, Kedves K, Araujo MG, Hoeflich A, Metzger F, Heppelmann M, Muscher-Banse A, Wrenzycki C, Pfarrer C, Schuberth HJ, Hoedemaker M, Bollwein H, Kaske M. Hepatic mRNA expression of acid labile subunit and deiodinase 1 differs between cows selected for high versus low concentrations of insulin-like growth factor 1 in late pregnancy. J Dairy Sci. 2013; 96:3737–3749.
Article25. Piechotta M, Mysegades W, Mense K, Meyerholz M, Hoedemaker M, Bollwein H. Plasma IGF-I concentrations in healthy ante partal dairy cows are indicative for development of a ketosis post partum. In : 8th ECBHM symposium; Buiatrissima. Bern: 2013. p. 209.26. Piechotta M, Sander AK, Kastelic JP, Wilde R, Heppelmann M, Rudolphi B, Schuberth HJ, Bollwein H, Kaske M. Short communication: Prepartum plasma insulin-like growth factor-I concentrations based on day of insemination are lower in cows developing postpartum diseases. J Dairy Sci. 2012; 95:1367–1370.
Article27. Radcliff RP, McCormack BL, Crooker BA, Lucy MC. Growth hormone (GH) binding and expression of GH receptor 1A mRNA in hepatic tissue of periparturient dairy cows. J Dairy Sci. 2003; 86:3933–3940.
Article28. Radcliff RP, McCormack BL, Keisler DH, Crooker BA, Lucy MC. Partial feed restriction decreases growth hormone receptor 1A mRNA expression in postpartum dairy cows. J Dairy Sci. 2006; 89:611–619.
Article29. Rhoads RP, Kim JW, Van Amburgh ME, Ehrhardt RA, Frank SJ, Boisclair YR. Effect of nutrition on the GH responsiveness of liver and adipose tissue in dairy cows. J Endocrinol. 2007; 195:49–58.
Article30. Rodriguez-Arnao J, Miell JP, Ross RJ. Influence of thyroid hormones on the GH-IGF-I axis. Trends Endocrinol Metab. 1993; 4:169–173.
Article31. Roh SG, Matsunaga N, Miyamoto A, Hidaka S, Hidari H. Competitive enzyme immunoassay for bovine growth hormone. Endocr J. 1997; 44:195–198.
Article32. Sandholm M. A preliminary report of a rapid method for the demonstration of abnormal gammaglobulin levels in bovine whole blood. Res Vet Sci. 1974; 17:32–35.
Article33. van Dorland HA, Richter S, Morel I, Doherr MG, Castro N, Bruckmaier RM. Variation in hepatic regulation of metabolism during the dry period and in early lactation in dairy cows. J Dairy Sci. 2009; 92:1924–1940.
Article34. Winkelman LA, Lucy MC, Elsasser TH, Pate JL, Reynolds CK. Short communication: Suppressor of cytokine signaling-2 mRNA increases after parturition in the liver of dairy cows. J Dairy Sci. 2008; 91:1080–1086.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk factors for repeat breeder dairy cows and their impacts on reproductive performance
- Ultrasonographic ovarian dynamic, plasma progesterone, and non-esterified fatty acids in lame postpartum dairy cows
- Relationship of Insulin like Growth Factor I with Pharmacologically Stimulated Growth Hormone Secretion in Growth Hormone Deficient Children
- Effect of resynchronization programs according to the ovarian status on pregnancy outcomes in dairy cows
- Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows