J Korean Med Sci.
2015 May;30(5):606-611. 10.3346/jkms.2015.30.5.606.
Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Acute Cholecystitis
- Affiliations
-
- 1Department of Hepatobiliary Surgery, Chonnam National University Medical School and Hospital, Gwangju, Korea.
- 2Department of Rheumatology, Chonnam National University Medical School and Hospital, Gwangju, Korea. parkyw@jnu.ac.kr
- 3Department of Pulmonary and Critical Care Medicine, Chonnam National University Medical School and Hospital, Gwangju, Korea.
- 4Department of Laboratory Medicine, Chonnam National University Medical School and Hospital, Gwangju, Korea.
- KMID: 2155474
- DOI: http://doi.org/10.3346/jkms.2015.30.5.606
Abstract
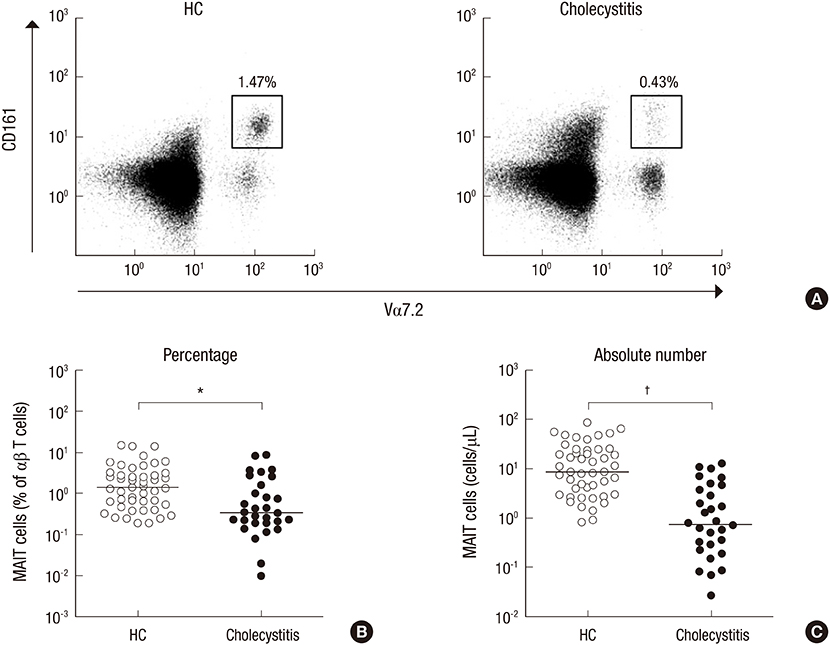
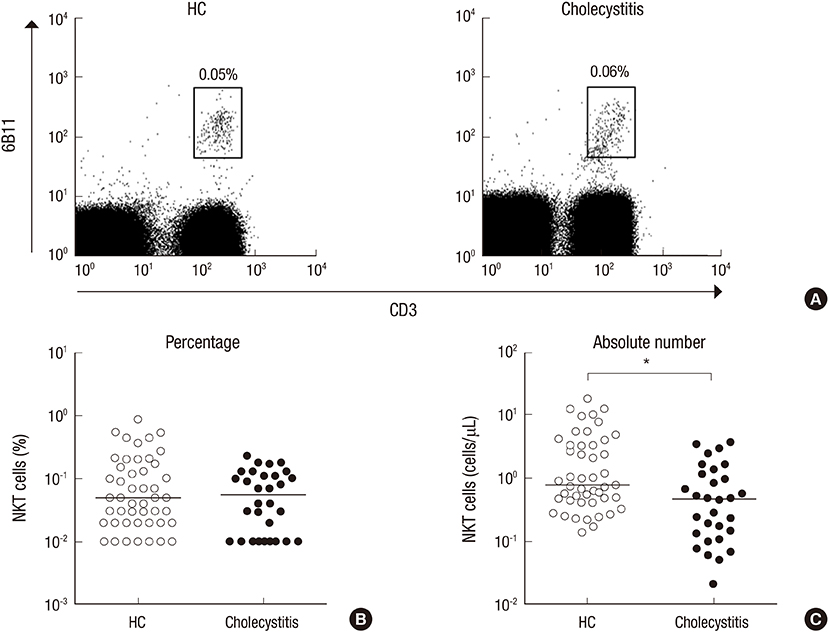
- Mucosal-associated invariant T (MAIT) cells and natural killer T (NKT) cells are known to play crucial roles in a variety of diseases, including autoimmunity, infectious diseases, and cancers. However, little is known about the roles of these invariant T cells in acute cholecystitis. The purposes of this study were to examine the levels of MAIT cells and NKT cells in patients with acute cholecystitis and to investigate potential relationships between clinical parameters and these cell levels. Thirty patients with pathologically proven acute cholecystitis and 47 age- and sex-matched healthy controls were enrolled. Disease grades were classified according to the revised Tokyo guidelines (TG13) for the severity assessment for acute cholecystitis. Levels of MAIT and NKT cells in peripheral blood were measured by flow cytometry. Circulating MAIT and NKT cell numbers were significantly lower in acute cholecystitis patients than in healthy controls, and these deficiencies in MAIT cells and NKT cell numbers were associated with aging in acute cholecystitis patients. Notably, a reduction in NKT cell numbers was found to be associated with severe TG13 grade, death, and high blood urea nitrogen levels. The study shows numerical deficiencies of circulating MAIT and NKT cells and age-related decline of these invariant T cells. In addition, NKT cell deficiency was associated with acute cholecystitis severity and outcome. These findings provide an information regarding the monitoring of these changes in circulating MAIT and NKT cell numbers during the course of acute cholecystitis and predicting prognosis.
MeSH Terms
-
Aged
Antibodies, Monoclonal/immunology
Case-Control Studies
Cholecystitis, Acute/*diagnosis/immunology/pathology
Female
Flow Cytometry
Humans
Leukocytes, Mononuclear/cytology
Male
Middle Aged
Natural Killer T-Cells/*cytology/immunology
Patients
Prognosis
Severity of Illness Index
T-Lymphocyte Subsets/*cytology/immunology
Antibodies, Monoclonal
Figure
Cited by 1 articles
-
Mucosal-associated Invariant T cells: A New Player in Innate Immunity
Yong-Wook Park, Seung-Jung Kee
J Rheum Dis. 2015;22(6):337-345. doi: 10.4078/jrd.2015.22.6.337.
Reference
-
1. Kimura Y, Takada T, Strasberg SM, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Windsor JA, Mayumi T, Yoshida M, et al. TG13 current terminology, etiology, and epidemiology of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013; 20:8–23.2. Lee SW, Yang SS, Chang CS, Yeh HJ. Impact of the Tokyo guidelines on the management of patients with acute calculous cholecystitis. J Gastroenterol Hepatol. 2009; 24:1857–1861.3. Wang AJ, Wang TE, Lin CC, Lin SC, Shih SC. Clinical predictors of severe gallbladder complications in acute acalculous cholecystitis. World J Gastroenterol. 2003; 9:2821–2823.4. Bondarev VI, Golovnia PF, Bondarenko SI, Radomskiĭ VT. BelonenkoGA. Disorders of immunoreactivity in acute cholecystitis in middle-aged and elderly patients and their correction in preventing postoperative complications. Klin Khir. 1990; 27–29.5. Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994; 180:1097–1106.6. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003; 422:164–169.7. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012; 491:717–723.8. Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005; 5:31–42.9. Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011; 32:212–218.10. Le Bourhis L, Mburu YK, Lantz O. MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol. 2013; 25:174–180.11. Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004; 114:1379–1388.12. Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Core M, Lévy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010; 11:701–708.13. Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008; 4:e1000239.14. Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011; 117:1250–1259.15. Kenna T, Golden-Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O'Farrelly C, Doherty DG. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003; 171:1775–1779.16. Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, et al. Tokyo Guidelines Revision Committee. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012; 19:578–585.17. Cho YN, Kee SJ, Lee SJ, Seo SR, Kim TJ, Lee SS, Kim MS, Lee WW, Yoo DH, Kim N, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology (Oxford). 2011; 50:1054–1063.18. Kee SJ, Kwon YS, Park YW, Cho YN, Lee SJ, Kim TJ, Lee SS, Jang HC, Shin MG, Shin JH, et al. Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun. 2012; 80:2100–2108.19. Lee SJ, Cho YN, Kim TJ, Park SC, Park DJ, Jin HM, Lee SS, Kee SJ, Kim N, Yoo DH, et al. Natural killer T cell deficiency in active adult-onset Still's Disease: correlation of deficiency of natural killer T cells with dysfunction of natural killer cells. Arthritis Rheum. 2012; 64:2868–2877.20. Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, Park KJ, Lee SJ, Lee SS, Kwon YS, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol. 2014; 193:3891–3901.21. Lee OJ, Cho YN, Kee SJ, Kim MJ, Jin HM, Lee SJ, Park KJ, Kim TJ, Lee SS, Kwon YS, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014; 49:47–54.22. Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol. 2005; 174:277–283.23. Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006; 107:3447–3454.24. Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006; 1072:52–61.25. Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014; 176:266–274.26. Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, Su J, Zhai F, Wang R, Zhang G, et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med. 2014; 190:329–339.27. Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007; 25:862–868.28. Molling JW, Kölgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, et al. Peripheral blood IFN-gamma-secreting Valpha24+ Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005; 116:87–93.29. Kee SJ, Park YW, Cho YN, Jin HM, Kim MJ, Lee SJ, Kim TJ, Lee SS, Kwon YS, Jang HC, et al. Age- and gender-related differences in circulating natural killer T cells and their subset levels in healthy Korean adults. Hum Immunol. 2012; 73:1011–1016.30. Brand JM, Kirchner H, Poppe C, Schmucker P. The effects of general anesthesia on human peripheral immune cell distribution and cytokine production. Clin Immunol Immunopathol. 1997; 83:190–194.31. Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Tillie JM, Dhabhar FS. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am. 2009; 91:2783–2794.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Multiple Trauma
- Altered Frequency, Activation, and Clinical Relevance of Circulating Innate and Innate-Like Lymphocytes in Patients With Alcoholic Liver Cirrhosis
- Intracellular Bacterial Infection and Invariant NKT Cells
- Comparison of Invariant NKT Cells with Conventional T Cells by Using Gene Set Enrichment Analysis (GSEA)
- Increased Th2-like Invariant Natural Killer T cells in Peripheral Blood From Patients With Asthma