J Korean Med Sci.
2015 May;30(5):576-585. 10.3346/jkms.2015.30.5.576.
The Effect of Umbilical Cord Blood Derived Mesenchymal Stem Cells in Monocrotaline-induced Pulmonary Artery Hypertension Rats
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. ymhong@ewha.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- 4Biomedical Research Institute, MEDIPOST, Co., Seoul, Korea.
- 5Division of Integrative Bioscience and Bioengineering, Pohang University of Science and Technology, Pohang, Korea.
- 6Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology, Pohang, Korea.
- KMID: 2155470
- DOI: http://doi.org/10.3346/jkms.2015.30.5.576
Abstract
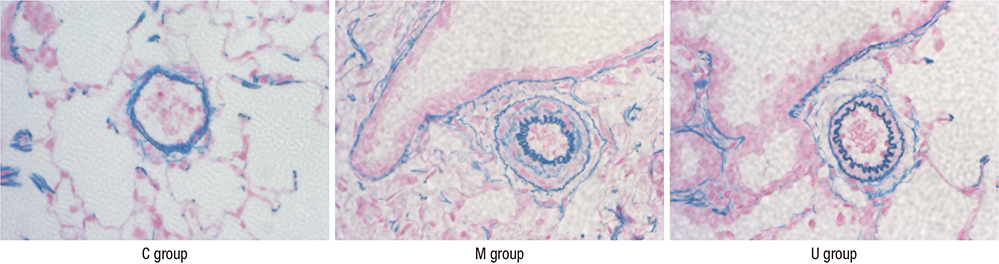
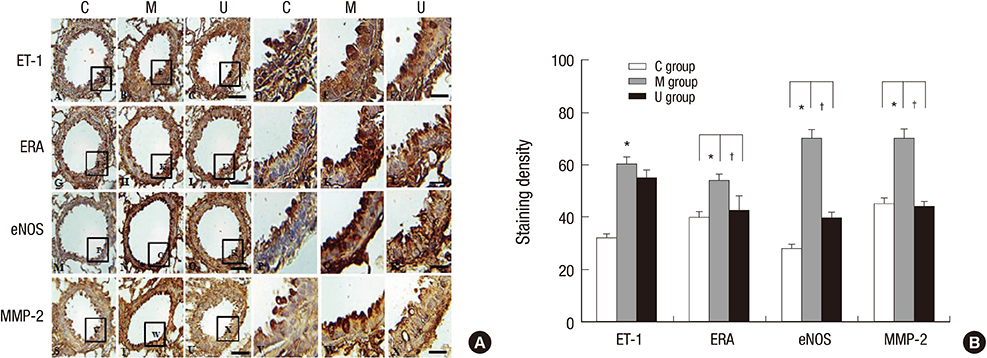
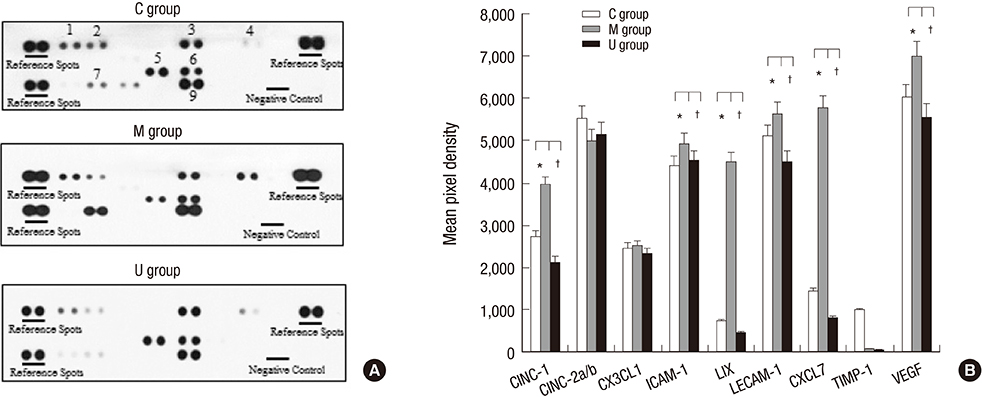
- Pulmonary arterial hypertension (PAH) causes right ventricular failure due to a gradual increase in pulmonary vascular resistance. The purposes of this study were to confirm the engraftment of human umbilical cord blood-mesenchymal stem cells (hUCB-MSCs) placed in the correct place in the lung and research on changes of hemodynamics, pulmonary pathology, immunomodulation and several gene expressions in monocrotaline (MCT)-induced PAH rat models after hUCB-MSCs transfusion. The rats were grouped as follows: the control (C) group; the M group (MCT 60 mg/kg); the U group (hUCB-MSCs transfusion). They received transfusions via the external jugular vein a week after MCT injection. The mean right ventricular pressure (RVP) was significantly reduced in the U group after the 2 week. The indicators of RV hypertrophy were significantly reduced in the U group at week 4. Reduced medial wall thickness in the pulmonary arteriole was noted in the U group at week 4. Reduced number of intra-acinar muscular pulmonary arteries was observed in the U group after 2 week. Protein expressions such as endothelin (ET)-1, endothelin receptor A (ERA), endothelial nitric oxide synthase (eNOS) and matrix metalloproteinase (MMP)-2 significantly decreased at week 4. The decreased levels of ERA, eNOS and MMP-2 immunoreactivity were noted by immnohistochemical staining. After hUCB-MSCs were administered, there were the improvement of RVH and mean RVP. Reductions in several protein expressions and immunomodulation were also detected. It is suggested that hUCB-MSCs may be a promising therapeutic option for PAH.
MeSH Terms
-
Animals
Cytokines/metabolism
Disease Models, Animal
Endothelin-1/metabolism
Fetal Blood/*cytology
Gene Expression Regulation/drug effects
Hemodynamics
Humans
Hypertension, Pulmonary/chemically induced/*therapy
Hypertrophy, Right Ventricular/physiopathology
Immunohistochemistry
Lung/metabolism/pathology
Male
Matrix Metalloproteinase 2/metabolism
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/*cytology/metabolism
Monocrotaline/toxicity
Nitric Oxide Synthase Type III/metabolism
Pulmonary Artery/pathology
Rats
Rats, Sprague-Dawley
Receptor, Endothelin A/metabolism
Cytokines
Endothelin-1
Monocrotaline
Matrix Metalloproteinase 2
Nitric Oxide Synthase Type III
Receptor, Endothelin A
Figure
Cited by 2 articles
-
Optimal Dose and Timing of Umbilical Stem Cells Treatment in Pulmonary Arterial Hypertensive Rats
Hyeryon Lee, Kwan Chang Kim, Soo Jin Choi, Young Mi Hong
Yonsei Med J. 2017;58(3):570-580. doi: 10.3349/ymj.2017.58.3.570.rBMSCs/ITGA5B1 Promotes Human Vascular Smooth Muscle Cell Differentiation via Enhancing Nitric Oxide Production
Yingxin Zhang, Jie Ding, Cong Xu, Hongli Yang, Peng Xia, Shengjun Ma, Haiying Chen
Int J Stem Cells. 2018;11(2):168-176. doi: 10.15283/ijsc18079.
Reference
-
1. Martin KB, Klinger JR, Rounds SI. Pulmonary arterial hypertension: new insights and new hope. Respirology. 2006; 11:6–17.2. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749.3. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004; 109:159–165.4. Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995; 333:214–221.5. Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol. 1998; 185:313–318.6. Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001; 104:424–428.7. Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L363–L369.8. Ryan J, Bloch K, Archer SL. Rodent models of pulmonary hypertension: harmonisation with the world health organisation's categorisation of human PH. Int J Clin Pract Suppl. 2011; 15–34.9. Beppu H. Transgenic and knockout mouse models for pulmonary hypertension: role of BMPR2: Part I: Genetically manipulated mouse models of pulmonary vascular disease and lung injury. Drug Discov Today. 2010; 7:61–65.10. Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014; 446:983–989.11. Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012; 98:465–473.12. Koo HS, Kim KC, Hong YM. Gene expressions of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011; 41:83–90.13. Kim KC, Lee HR, Kim SJ, Cho MS, Hong YM. Changes of gene expression after bone marrow cell transfusion in rats with monocrotaline-induced pulmonary hypertension. J Korean Med Sci. 2012; 27:605–613.14. Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, Choi S, Oh W, Yang YS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004; 6:476–486.15. O'Callaghan DS, Savale L, Yaïci A, Natali D, Jaïs X, Parent F, Montani D, Humbert M, Simonneau G, Sitbon O. Endothelin receptor antagonists for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2011; 12:1585–1596.16. Zhang H, Fazel S, Tian H, Mickle DA, Weisel RD, Fujii T, Li RK. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005; 289:H2089–H2096.17. Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001; 97:2962–2971.18. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004; 4:11–22.19. Munoz RM, Han H, Tegeler T, Petritis K, Von Hoff DD, Hoffman SA. Isolation and characterization of muscle fatigue substance with anti-tumor activities. J Cancer. 2013; 4:343–349.20. Duncan M, Wagner BD, Murray K, Allen J, Colvin K, Accurso FJ, Ivy DD. Circulating cytokines and growth factors in pediatric pulmonary hypertension. Mediators Inflamm. 2012; 2012:143428.21. Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation. 2007; 115:509–517.22. Angelini A, Castellani C, Ravara B, Franzin C, Pozzobon M, Tavano R, Libera LD, Papini E, Vettor R, De Coppi P, et al. Stem-cell therapy in an experimental model of pulmonary hypertension and right heart failure: role of paracrine and neurohormonal milieu in the remodeling process. J Heart Lung Transplant. 2011; 30:1281–1293.23. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000; 109:235–242.24. Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik's cube of cell therapy for heart disease. Dis Model Mech. 2009; 2:344–358.25. Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008; 177:701–711.26. Wu KH, Zhou B, Lu SH, Feng B, Yang SG, Du WT, Gu DS, Han ZC, Liu YL. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007; 100:608–616.27. Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011; 7:195–207.28. Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006; 173:171–179.29. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–3506.30. Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009; 18:869–886.31. Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007; 292:H1120–H1128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microarray analysis after umbilical cord blood derived mesenchymal stem cells injection in monocrotaline-induced pulmonary artery hypertension rats
- Optimal Dose and Timing of Umbilical Stem Cells Treatment in Pulmonary Arterial Hypertensive Rats
- Therapeutic Effects of Umbilical Cord Blood Derived Mesenchymal Stem Cell-Conditioned Medium on Pulmonary Arterial Hypertension in Rats
- Changes in Caspase-3, B Cell Leukemia/Lymphoma-2, Interleukin-6, Tumor Necrosis Factor-α and Vascular Endothelial Growth Factor Gene Expression after Human Umbilical Cord Blood Derived Mesenchymal Stem Cells Transfusion in Pulmonary Hypertension Rat Models
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood