Ann Surg Treat Res.
2016 Mar;90(3):124-130. 10.4174/astr.2016.90.3.124.
Validation of international consensus guideline 2012 for intraductal papillary mucinous neoplasm of pancreas
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dwchoi@skku.edu
- KMID: 2155045
- DOI: http://doi.org/10.4174/astr.2016.90.3.124
Abstract
- PURPOSE
Intraductal papillary mucinous neoplasm (IPMN) has variable malignant potential ranging from premalignant intraductal lesions to malignant neoplasms with invasive carcinoma. To help physicians managing patients with IPMN, International consensus guidelines was made in 2006 and revised in 2012. This study was designed to evaluate the clinical usefulness of guidelines and to validate.
METHODS
From October 1996 to December 2011, we retrospectively reviewed the data of 230 patients who underwent pancreatic resection for IPMN. Univariate and multivariable analyses were used to identify significant predictors of malignancy in IPMN.
RESULTS
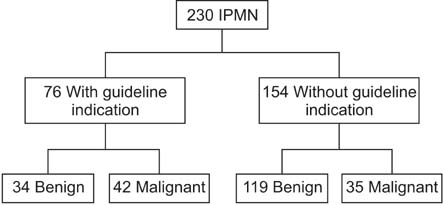
Of the 230 patients, 62 patients (27%) were diagnosed with invasive carcinoma. Jaundice (P < 0.001; 95% confidence interval [CI], 3.086-40.010) main pancreatic duct diameter equal to or greater than 10 mm (P < 0.001; 95% CI, 1.723-6.673) and also abdominal pain (P < 0.001; 95% CI, 4.363-22.600) show statistical significance in univariate and multivariate analysis. "High-risk stigmata" was statistical powerful predictors of malignancy than "worrisome features". International consensus guidelines 2012 had improvement on specificity but deterioration of sensitivity.
CONCLUSION
Revised guidelines seemed to bring about an improvement of weak side of Sendai criteria. Abdominal pain, jaundice, main pancreas duct greater than 10 mm can be clinical variables to predict malignancy.
MeSH Terms
Figure
Reference
-
1. Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010; 139:708–713. 713.e1–713.e2.2. Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995; 18:197–206.3. Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003; 138:427–423.4. Grutzmann R, Post S, Saeger HD, Niedergethmann M. Intraductal papillary mucinous neoplasia (IPMN) of the pancreas: its diagnosis, treatment, and prognosis. Dtsch Arztebl Int. 2011; 108:788–794.5. Swartz MJ, Hsu CC, Pawlik TM, Winter J, Hruban RH, Guler M, et al. Adjuvant chemoradiotherapy after pancreatic resection for invasive carcinoma associated with intraductal papillary mucinous neoplasm of the pancreas. Int J Radiat Oncol Biol Phys. 2010; 76:839–844.6. Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012; 12:183–197.7. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6:17–32.8. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004; 351:1218–1226.9. Akita H, Takeda Y, Hoshino H, Wada H, Kobayashi S, Marubashi S, et al. Mural nodule in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am J Surg. 2011; 202:214–219.10. Maguchi H, Tanno S, Mizuno N, Hanada K, Kobayashi G, Hatori T, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011; 40:364–370.11. Nagai K, Doi R, Ito T, Kida A, Koizumi M, Masui T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009; 16:353–358.12. Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007; 102:1759–1764.13. Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, et al. Branchduct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007; 133:72–79.14. Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007; 56:1086–1090.15. Tang RS, Weinberg B, Dawson DW, Reber H, Hines OJ, Tomlinson JS, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008; 6:815–819.16. Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008; 57:339–343.17. Uehara H, Ishikawa O, Katayama K, Kawada N, Ikezawa K, Fukutake N, et al. Size of mural nodule as an indicator of surgery for branch duct intraductal papillary mucinous neoplasm of the pancreas during follow-up. J Gastroenterol. 2011; 46:657–663.18. Woo SM, Ryu JK, Lee SH, Yoon WJ, Kim YT, Yoon YB. Branch duct intraductal papillary mucinous neoplasms in a retrospective series of 190 patients. Br J Surg. 2009; 96:405–411.19. Jang JY, Kim SW, Lee SE, Yang SH, Lee KU, Lee YJ, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008; 15:199–205.20. Sawhney MS, Al-Bashir S, Cury MS, Brown A, Chuttani R, Pleskow DK, et al. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. 2009; 7:1373–1376.21. Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008; 144:677–684.22. Aso T, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, et al. "High-risk stigmata" of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2014; 43:1239–1243.23. Goh BK, Thng CH, Tan DM, Low AS, Wong JS, Cheow PC, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg. 2014; 208:202–209.24. Jang JY, Park T, Lee S, Kang MJ, Lee SY, Lee KB, et al. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014; 101:686–692.25. Roch AM, Ceppa EP, DeWitt JM, Al-Haddad MA, House MG, Nakeeb A, et al. International Consensus Guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB (Oxford). 2014; 16:929–935.26. Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013; 258:466–475.27. Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, Ideno N, et al. Role of pancreatic juice cytology in the preoperative management of intraductal papillary mucinous neoplasm of the pancreas in the era of international consensus guidelines 2012. World J Surg. 2014; 38:2994–3001.28. Goh BK, Lin Z, Tan DM, Thng CH, Khor CJ, Lim TK, et al. Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: Results from a systematic review of 1,382 surgically resected patients. Surgery. 2015; 158:1192–1202.29. Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, et al. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012; 255:517–522.30. Maire F, Voitot H, Aubert A, Palazzo L, O'Toole D, Couvelard A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008; 103:2871–2877.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of malignant intraductal papillary mucinous neoplasms of the pancreas on computed tomography and magnetic resonance imaging
- Oncocytic Type Intraductal Papillary Mucinous Neoplasm Mimicking Mucinous Cystic Neoplasm of the Pancreas: A Case Report
- Intraductal Papillary Mucinous Tumor Simultaneously Involving the Liver and Pancreas: A Case Report
- A Case of Intraductal Papillary Mucinous Tumor of the Pancreas with Scant Mucin Production
- Cystic Neoplasms and Intraductal Papillary Mucinous Neoplasms of the Pancreas