Immune Netw.
2016 Feb;16(1):75-84. 10.4110/in.2016.16.1.75.
Immunogenic Cell Death Induced by Ginsenoside Rg3: Significance in Dendritic Cell-based Anti-tumor Immunotherapy
- Affiliations
-
- 1R&D Center, Pharmicell Co. Ltd., Seongnam 13229, Korea. andyjosh@pharmicell.com
- 2Department of Thoracic and Cardiovascular Surgery, Jeju National University School of Medicine, Jeju 63241, Korea.
- KMID: 2154813
- DOI: http://doi.org/10.4110/in.2016.16.1.75
Abstract
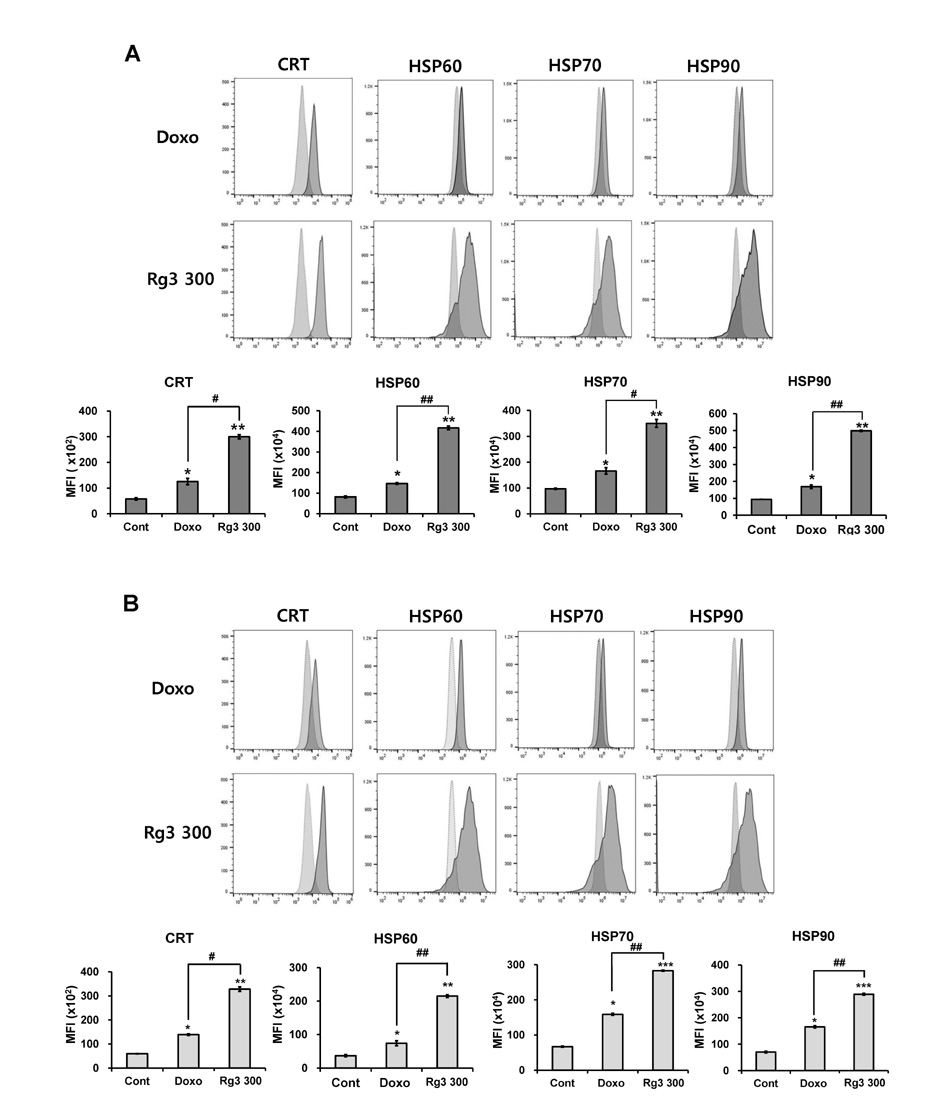
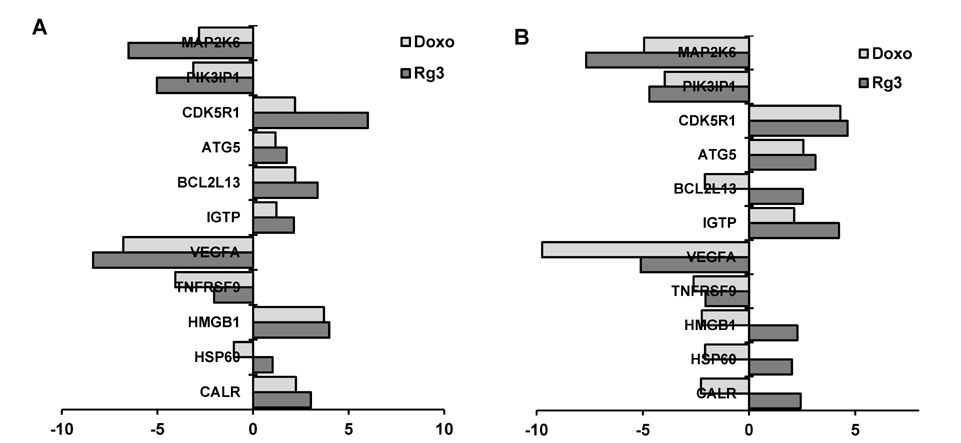
- Cancer is one of the leading causes of morbidity and mortality worldwide; therefore there is a need to discover new therapeutic modules with improved efficacy and safety. Immune-(cell) therapy is a promising therapeutic strategy for the treatment of intractable cancers. The effectiveness of certain chemotherapeutics in inducing immunogenic tumor cell death thus promoting cancer eradication has been reported. Ginsenoside Rg3 is a ginseng saponin that has antitumor and immunomodulatory activity. In this study, we treated tumor cells with Rg3 to verify the significance of inducing immunogenic tumor cell death in antitumor therapy, especially in DC-based immunotherapy. Rg3 killed the both immunogenic (B16F10 melanoma cells) and non-immunogenic (LLC: Lewis Lung Carcinoma cells) tumor cells by inducing apoptosis. Surface expression of immunogenic death markers including calreticulin and heat shock proteins and the transcription of relevant genes were increased in the Rg3-dying tumor. Increased calreticulin expression was directly related to the uptake of dying tumor cells by dendritic cells (DCs): the proportion of CRT+ CD11c+ cells was increased in the Rg3-treated group. Interestingly, tumor cells dying by immunogenic cell death secreted IFN-gamma, an effector molecule for antitumor activity in T cells. Along with the Rg3-induced suppression of pro-angiogenic (TNF-alpha) and immunosuppressive cytokine (TGF-beta) secretion, IFN-gamma production from the Rg3-treated tumor cells may also indicate Rg3 as an effective anticancer immunotherapeutic strategy. The data clearly suggests that Rg3-induced immunogenic tumor cell death due its cytotoxic effect and its ability to induce DC function. This indicates that Rg3 may be an effective immunotherapeutic strategy.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis
Se Jin Oh, Jaeyoon Lee, Yukang Kim, Kwon-Ho Song, Eunho Cho, Minsung Kim, Heejae Jung, Tae Woo Kim
Immune Netw. 2020;20(1):e7. doi: 10.4110/in.2020.20.e7.Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis
Se Jin Oh, Jaeyoon Lee, Yukang Kim, Kwon-Ho Song, Eunho Cho, Minsung Kim, Tae Woo Kim, Heejae Jung
Immune Netw. 2020;20(1):. doi: 10.4110/in.2020.20.e7.
Reference
-
1. Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM, Lee BY, Kwon SM. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012; 13:504–515.
Article2. Nam KY, Choi JE, Hong SC, Pyo KM, Park JD. Recent Progress in Research on Anticancer Activities of Ginsenoside-Rg3. Korean J Pharmacogn. 2014; 45:1–10.3. Cirone M, Di RL, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Activation of dendritic cells by tumor cell death. Oncoimmunology. 2012; 1:1218–1219.
Article4. Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011; 71:4821–4833.
Article5. Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010; 16:3100–3104.
Article6. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van EP, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009; 28:578–590.
Article7. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van EP, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007; 13:54–61.
Article8. Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997; 60:52–60.
Article9. Joo EJ, Ha YW, Shin H, Son SH, Kim YS. Generation and characterization of monoclonal antibody to ginsenoside rg3. Biol Pharm Bull. 2009; 32:548–552.
Article10. Gao JL, Lv GY, He BC, Zhang BQ, Zhang H, Wang N, Wang CZ, Du W, Yuan CS, He TC. Ginseng saponin metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncol Rep. 2013; 30:292–298.
Article11. Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007; 73:669–674.
Article12. Liu TG, Huang Y, Cui DD, Huang XB, Mao SH, Ji LL, Song HB, Yi C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009; 9:250.
Article13. Zhang Q, Kang X, Zhao W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Commun. 2006; 342:824–828.
Article14. Yi C, Huang XB, Hou M. [Experimental study on effect of chemotherapy combined ginsengnoside Rg3 in treating pulmonary carcinoma]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005; 25:58–59.15. Hu SS, Zhou LK, Ba Y, Li HI, Zhu CH. A meta-analysis of Ginsenoside Rg3 for non-small cell lung cancer. Clin Oncol Cancer Res. 2011; 8:175–180.
Article16. Liu JW, Chen JX, Yu LH, Tian YX, Cui XY, Yan Q, Fu L. [Inhibitory effect of ginsenoside-Rg3 on lung metastasis of mouse melanoma transfected with ribonuclease inhibitor]. Zhonghua Zhong Liu Za Zhi. 2004; 26:722–725.17. Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su MM, Wang DD, Wang WJ. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl). 2008; 121:1394–1397.
Article18. Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K, Kim TJ, Ham J, Jang HJ, Kang KS, Ko H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014; 322:23–33.
Article19. Lee SG, Kim BS, Nam JO. Ginsenoside Rg3 induces apoptosis in B16F10 melonoma cells. J Life Sci. 2014; 24:1001–1005.20. Oh SJ, Ryu CK, Baek SY, Lee H. Cellular mechanism of newly synthesized indoledione derivative-induced immunological death of tumor cell. Immune Netw. 2011; 11:383–389.
Article21. Park D, Bae DK, Jeon JH, Lee J, Oh N, Yang G, Yang YH, Kim TK, Song J, Lee SH, Song BS, Jeon TH, Kang SJ, Joo SS, Kim SU, Kim YB. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011; 31:397–405.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Production of Ginsenoside-Rg3 from Lipomyces starkeyi Grown on Ginseng-Steaming Effluent
- Ginsenoside Rg3 attenuates ischemia reperfusion induced renal injury in mice via induction of autophagy flux
- B Cells Transduced with HPV16 E6/E7-expressing Adenoviral Vector Can Efficiently Induce CTL-dependent Anti-Tumor Immunity
- Current Approaches in Development of Immunotherapeutic Vaccines for Breast Cancer
- Anti-cancer Activities of Ginseng Extract Fermented with Phellinus linteus