Immune Netw.
2016 Feb;16(1):52-60. 10.4110/in.2016.16.1.52.
Generation of Tolerogenic Dendritic Cells and Their Therapeutic Applications
- Affiliations
-
- 1System Immunology Laboratory, Department of Biochemistry, College of Life Science & Biotechnology, Yonsei University, Seoul 03722, Korea. sjha@yonsei.ac.kr
- KMID: 2154811
- DOI: http://doi.org/10.4110/in.2016.16.1.52
Abstract
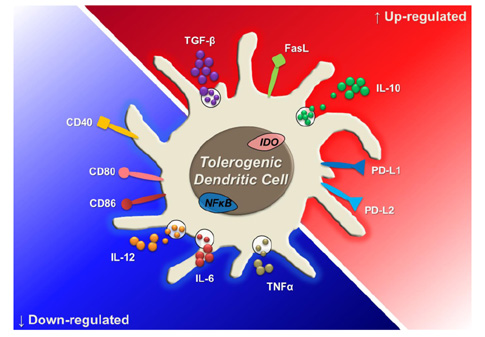
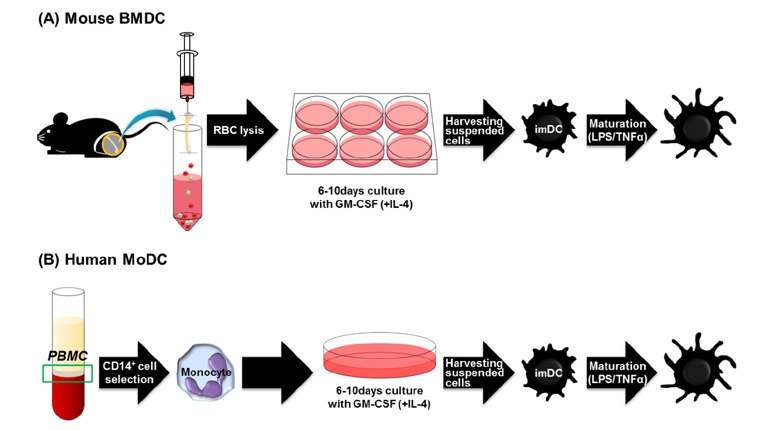
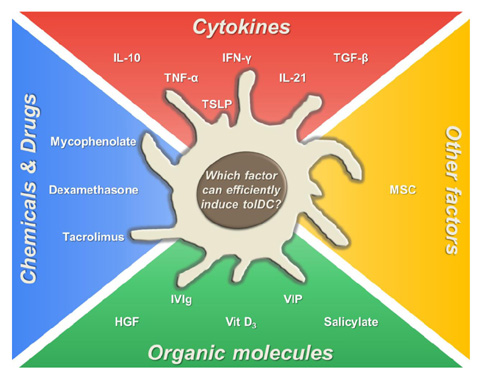
- Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that bridge innate and adaptive immune responses, thereby leading to immune activation. DCs have been known to recognize pathogen-associated molecular patterns such as lipopolysaccharides (LPS) and nucleic acids via their pattern recognition receptors, which trigger signaling of their maturation and effector functions. Furthermore, DCs take up and process antigens as a form of peptide loaded on the major histocompatibility complex (MHC) and present them to T cells, which are responsible for the adaptive immune response. Conversely, DCs can also play a role in inducing immune suppression under specific circumstances. From this perspective, the role of DCs is related to tolerance rather than immunity. Immunologists refer to these special DCs as tolerogenic DCs (tolDCs). However, the definition of tolDCs is controversial, and there is limited information on their development and characteristics. In this review, we discuss the current concept of tolDCs, cutting-edge methods for generating tolDCs in vitro, and future applications of tolDCs, including clinical use.
MeSH Terms
Figure
Cited by 3 articles
-
Generation, Characteristics and Clinical Trials of
Ex Vivo Generated Tolerogenic Dendritic Cells
Sang-Hyun Kim, Ho-Hyun Jung, Chong-Kil Lee
Yonsei Med J. 2018;59(7):807-815. doi: 10.3349/ymj.2018.59.7.807.Extended Culture of Bone Marrow with Granulocyte Macrophage-Colony Stimulating Factor Generates Immunosuppressive Cells
Hye Young Na, Moah Sohn, Seul Hye Ryu, Wanho Choi, Hyunju In, Hyun Soo Shin, Chae Gyu Park
Immune Netw. 2018;18(2):e16. doi: 10.4110/in.2018.18.e16.Extended Culture of Bone Marrow with Granulocyte Macrophage-Colony Stimulating Factor Generates Immunosuppressive Cells
Hye Young Na, Moah Sohn, Seul Hye Ryu, Wanho Choi, Hyunju In, Hyun Soo Shin, Chae Gyu Park
Immune Netw. 2018;18(2):. doi: 10.4110/in.2018.18.e16.
Reference
-
1. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192:1027–1034.
Article2. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001; 2:261–268.
Article3. Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006; 177:8291–8295.
Article4. Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006; 36:2472–2482.
Article5. Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003; 170:1257–1266.
Article6. Chen C, Qu QX, Huang JA, Zhu YB, Ge Y, Wang Q, Zhang XG. Expression of programmed-death receptor ligands 1 and 2 may contribute to the poor stimulatory potential of murine immature dendritic cells. Immunobiology. 2007; 212:159–165.
Article7. Chen L, Zhang Z, Chen W, Zhang Z, Li Y, Shi M, Zhang J, Chen L, Wang S, Wang FS. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol. 2007; 178:6634–6641.
Article8. Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003; 9:562–567.
Article9. Kim HK, Guan H, Zu G, Li H, Wu L, Feng X, Elmets C, Fu Y, Xu H. High-level expression of B7-H1 molecules by dendritic cells suppresses the function of activated T cells and desensitizes allergen-primed animals. J Leukoc Biol. 2006; 79:686–695.
Article10. Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004; 101:10691–10696.
Article11. Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003; 170:3637–3644.
Article12. Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, Thomson AW. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008; 85:369–377.
Article13. Waisman A, Yogev N. B7-H1 and CD8+ Treg: the enigmatic role of B7-H1 in peripheral tolerance. Eur J Immunol. 2009; 39:1448–1451.
Article14. King LB, Ashwell JD. Signaling for death of lymphoid cells. Curr Opin Immunol. 1993; 5:368–373.
Article15. Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993; 5:625–630.
Article16. Brunda MJ. Interleukin-12. J Leukoc Biol. 1994; 55:280–288.
Article17. Zlotnik A, Moore KW. Interleukin 10. Cytokine. 1991; 3:366–371.
Article18. Spits H, de Waal MR. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992; 99:8–15.
Article19. Taga K, Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992; 148:1143–1148.20. Rennick D, Berg D, Holland G. Interleukin 10: an overview. Prog Growth Factor Res. 1992; 4:207–227.
Article21. de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te VA, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991; 174:915–924.
Article22. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009; 9:447–453.
Article23. Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998; 9:315–323.
Article24. Xie FT, Cao JS, Zhao J, Yu Y, Qi F, Dai XC. IDO expressing dendritic cells suppress allograft rejection of small bowel transplantation in mice by expansion of Foxp3+ regulatory T cells. Transpl Immunol. 2015; 33:69–77.
Article25. Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000; 164:3596–3599.
Article26. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002; 196:459–468.
Article27. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002; 297:1867–1870.
Article28. Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002; 196:447–457.
Article29. van der Marel AP, Samsom JN, Greuter M, van Berkel LA, O'Toole T, Kraal G, Mebius RE. Blockade of IDO inhibits nasal tolerance induction. J Immunol. 2007; 179:894–900.
Article30. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992; 176:1693–1702.
Article31. Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993; 90:3038–3042.
Article32. Witmer-Pack MD, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987; 166:1484–1498.
Article33. Caux C, zutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992; 360:258–261.
Article34. Santiago-Schwarz F, Belilos E, Diamond B, Carsons SE. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992; 52:274–281.
Article35. Caux C, Vanbervliet B, Massacrier C, zutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996; 184:695–706.
Article36. Rosenzwajg M, Canque B, Gluckman JC. Human dendritic cell differentiation pathway from CD34+ hematopoietic precursor cells. Blood. 1996; 87:535–544.
Article37. Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992; 149:2681–2688.38. Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995; 154:5851–5861.39. Torres-Aguilar H, Blank M, Jara LJ, Shoenfeld Y. Tolerogenic dendritic cells in autoimmune diseases: crucial players in induction and prevention of autoimmunity. Autoimmun Rev. 2010; 10:8–17.40. Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten BA. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol. 2012; 142:332–342.
Article41. Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology. 2002; 107:489–499.
Article42. Muller G, Muller A, Tuting T, Steinbrink K, Saloga J, Szalma C, Knop J, Enk AH. Interleukin-10-treated dendritic cells modulate immune responses of naive and sensitized T cells in vivo. J Invest Dermatol. 2002; 119:836–841.43. De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997; 27:1229–1235.
Article44. Eljaafari A, Li YP, Miossec P. IFN-gamma, as secreted during an alloresponse, induces differentiation of monocytes into tolerogenic dendritic cells, resulting in FoxP3+ regulatory T cell promotion. J Immunol. 2009; 183:2932–2945.
Article45. Svajger U, Obermajer N, Jeras M. IFN-gamma-rich environment programs dendritic cells toward silencing of cytotoxic immune responses. J Leukoc Biol. 2014; 95:33–46.
Article46. Della Bella S, Mavilio D. IFN-gamma: a Janus-faced cytokine in dendritic cell programming. J Leukoc Biol. 2014; 95:6–8.47. Kerkar SP, Chinnasamy D, Hadi N, Melenhorst J, Muranski P, Spyridonidis A, Ito S, Weber G, Yin F, Hensel N, Wang E, Marincola FM, Barrett AJ. Timing and intensity of exposure to interferon-gamma critically determines the function of monocyte-derived dendritic cells. Immunology. 2014; 143:96–108.
Article48. Delneste Y, Charbonnier P, Herbault N, Magistrelli G, Caron G, Bonnefoy JY, Jeannin P. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003; 101:143–150.
Article49. Thomas DC, Wong FS, Zaccone P, Green EA, Wallberg M. Protection of islet grafts through transforming growth factor-beta-induced tolerogenic dendritic cells. Diabetes. 2013; 62:3132–3142.
Article50. O'Flynn L, Treacy O, Ryan AE, Morcos M, Cregg M, Gerlach J, Joshi L, Nosov M, Ritter T. Donor bone marrow-derived dendritic cells prolong corneal allograft survival and promote an intragraft immunoregulatory milieu. Mol Ther. 2013; 21:2102–2112.51. Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang XN, Isaacs JD, Hilkens CM. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008; 84:124–133.
Article52. Anderson AE, Swan DJ, Sayers BL, Harry RA, Patterson AM, von Delwig A, Robinson JH, Isaacs JD, Hilkens CM. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Biol. 2009; 85:243–250.
Article53. Harry RA, Anderson AE, Isaacs JD, Hilkens CM. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis. 2010; 69:2042–2050.
Article54. Bosma BM, Metselaar HJ, Nagtzaam NM, de Haan R, Mancham S, van der Laan LJ, Kuipers EJ, Kwekkeboom J. Dexamethasone transforms lipopolysaccharide-stimulated human blood myeloid dendritic cells into myeloid dendritic cells that prime interleukin-10 production in T cells. Immunology. 2008; 125:91–100.
Article55. Ferreira GB, van Etten E, Verstuyf A, Waer M, Overbergh L, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab Res Rev. 2011; 27:933–941.
Article56. Matsuzaki J, Tsuji T, Zhang Y, Wakita D, Imazeki I, Sakai T, Ikeda H, Nishimura T. 1alpha,25-Dihydroxyvitamin D3 downmodulates the functional differentiation of Th1 cytokine-conditioned bone marrow-derived dendritic cells beneficial for cytotoxic T lymphocyte generation. Cancer Sci. 2006; 97:139–147.
Article57. Farias AS, Spagnol GS, Bordeaux-Rego P, Oliveira CO, Fontana AG, de Paula RF, Santos MP, Pradella F, Moraes AS, Oliveira EC, Longhini AL, Rezende AC, Vaisberg MW, Santos LM. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci Ther. 2013; 19:269–277.
Article58. Nikolic T, Roep BO. Regulatory multitasking of tolerogenic dendritic cells - lessons taken from vitamin d3-treated tolerogenic dendritic cells. Front Immunol. 2013; 4:113.
Article59. Volchenkov R, Brun JG, Jonsson R, Appel S. In vitro suppression of immune responses using monocyte-derived tolerogenic dendritic cells from patients with primary Sjogren's syndrome. Arthritis Res Ther. 2013; 15:R114.60. Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005; 105:4120–4126.
Article61. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005; 105:2214–2219.
Article62. Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, Bartholomew A, Garcia B, Wang H. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant. 2009; 9:1760–1772.
Article63. Wang H, Qi F, Dai X, Tian W, Liu T, Han H, Zhang B, Li H, Zhang Z, Du C. Requirement of B7-H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transpl Immunol. 2014; 31:65–74.
Article64. Guyot P, Taylor P, Christensen R, Pericleous L, Poncet C, Lebmeier M, Drost P, Bergman G. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther. 2011; 13:R204.
Article65. Hilkens CM, Isaacs JD. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin Exp Immunol. 2013; 172:148–157.
Article66. Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009; 60:380–389.
Article67. Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci U S A. 2005; 102:13562–13567.
Article68. Healy LJ, Collins HL, Thompson SJ. Systemic administration of tolerogenic dendritic cells ameliorates murine inflammatory arthritis. Open Rheumatol J. 2008; 2:71–80.
Article69. Jaen O, Rulle S, Bessis N, Zago A, Boissier MC, Falgarone G. Dendritic cells modulated by innate immunity improve collagen-induced arthritis and induce regulatory T cells in vivo. Immunology. 2009; 126:35–44.
Article70. Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001; 166:3499–3505.
Article71. Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, Mule JJ, McDonagh KT, Fox DA. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001; 107:1275–1284.
Article72. Ning B, Wei J, Zhang A, Gong W, Fu J, Jia T, Yang SY. Antigen-specific tolerogenic dendritic cells ameliorate the severity of murine collagen-induced arthritis. PLoS One. 2015; 10:e0131152.
Article73. Popov I, Li M, Zheng X, San H, Zhang X, Ichim TE, Suzuki M, Feng B, Vladau C, Zhong R, Garcia B, Strejan G, Inman RD, Min WP. Preventing autoimmune arthritis using antigen-specific immature dendritic cells: a novel tolerogenic vaccine. Arthritis Res Ther. 2006; 8:R141.74. Salazar L, Aravena O, Abello P, Escobar A, Contreras-Levicoy J, Rojas-Colonelli N, Catalan D, Aguirre A, Zuniga R, Pesce B, Gonzalez C, Cepeda R, Cuchacovich M, Molina MC, Salazar-Onfray F, Delgado M, Toes RE, Aguillon JC. Modulation of established murine collagen-induced arthritis by a single inoculation of short-term lipopolysaccharide-stimulated dendritic cells. Ann Rheum Dis. 2008; 67:1235–1241.
Article75. Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997; 159:4772–4780.76. van Duivenvoorde LM, Han WG, Bakker AM, Louis-Plence P, Charbonnier LM, Apparailly F, van der Voort EI, Jorgensen V, Huizinga TW, Toes RE. Immunomodulatory dendritic cells inhibit Th1 responses and arthritis via different mechanisms. J Immunol. 2007; 179:1506–1515.
Article77. van Duivenvoorde LM, Louis-Plence P, Apparailly F, van der Voort EI, Huizinga TW, Jorgensen C, Toes RE. Antigen-specific immunomodulation of collagen-induced arthritis with tumor necrosis factor-stimulated dendritic cells. Arthritis Rheum. 2004; 50:3354–3364.
Article78. Xiao BG, Zhu WH, Lu CZ. The presence of GM-CSF and IL-4 interferes with effect of TGF-beta1 on antigen presenting cells in patients with multiple sclerosis and in rats with experimental autoimmune encephalomyelitis. Cell Immunol. 2007; 249:30–36.
Article79. Fu J, Zhang A, Ju X. Tolerogenic dendritic cells as a target for the therapy of immune thrombocytopenia. Clin Appl Thromb Hemost. 2012; 18:469–475.
Article80. Adorini L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann N Y Acad Sci. 2003; 987:258–261.
Article81. Lau AW, Biester S, Cornall RJ, Forrester JV. Lipopolysaccharide-activated IL-10-secreting dendritic cells suppress experimental autoimmune uveoretinitis by MHCII-dependent activation of CD62L-expressing regulatory T cells. J Immunol. 2008; 180:3889–3899.
Article82. Silva Pde M, Bier J, Paiatto LN, Galdino AC, Lopes SC, Fernandes LG, Tamashiro WM, Simioni PU. Tolerogenic Dendritic Cells on Transplantation: Immunotherapy Based on Second Signal Blockage. J Immunol Res. 2015; 2015:856707.83. Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011; 23:252–263.
Article84. Li GP, Yang J, Hao J, Yang YM, Ren YN, Xie RF, Fan HH, Qian KC. [The role of third-party tolerogenic dendritic cells in the prevention of acute graft-versus-host-disease following allogeneic bone marrow transplantation in mice]. Zhonghua Xue Ye Xue Za Zhi. 2012; 33:461–466.85. Moreau A, Varey E, Bouchet-Delbos L, Cuturi MC. Cell therapy using tolerogenic dendritic cells in transplantation. Transplant Res. 2012; 1:13.
Article86. Cobbold SP, Waldmann H. Regulatory cells and transplantation tolerance. Cold Spring Harb Perspect Med. 2013; 3:pii: a015545.
Article87. Moreau A, Varey E, Beriou G, Hill M, Bouchet-Delbos L, Segovia M, Cuturi MC. Tolerogenic dendritic cells and negative vaccination in transplantation: from rodents to clinical trials. Front Immunol. 2012; 3:218.
Article88. Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol. 2004; 173:4331–4341.
Article89. Stoop JN, Harry RA, von Delwig A, Isaacs JD, Robinson JH, Hilkens CM. Therapeutic effect of tolerogenic dendritic cells in established collagen-induced arthritis is associated with a reduction in Th17 responses. Arthritis Rheum. 2010; 62:3656–3665.
Article90. Morel PA, Turner MS. Dendritic cells and the maintenance of self-tolerance. Immunol Res. 2011; 50:124–129.
Article91. Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002; 23:445–449.
Article92. Dudek AM, Martin S, Garg AD, Agostinis P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front Immunol. 2013; 4:438.
Article93. Voigtlander C, Rossner S, Cierpka E, Theiner G, Wiethe C, Menges M, Schuler G, Lutz MB. Dendritic cells matured with TNF can be further activated in vitro and after subcutaneous injection in vivo which converts their tolerogenicity into immunogenicity. J Immunother. 2006; 29:407–415.94. Lim DS, Kang MS, Jeong JA, Bae YS. Semi-mature DC are immunogenic and not tolerogenic when inoculated at a high dose in collagen-induced arthritis mice. Eur J Immunol. 2009; 39:1334–1343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Generation, Characteristics and Clinical Trials of Ex Vivo Generated Tolerogenic Dendritic Cells
- Lactoferrin Induces Tolerogenic Bone Marrow-Derived Dendritic Cells
- Dendritic Cell-based Immunotherapy for Rheumatoid Arthritis: from Bench to Bedside
- 3,3'-Diindolylmethane Inhibits Flt3L/GM-CSF-induced-bone Marrow-derived CD103+ Dendritic Cell Differentiation Regulating Phosphorylation of STAT3 and STAT5
- Regulation of Th2 Cell Immunity by Dendritic Cells