Clin Exp Vaccine Res.
2016 Jan;5(1):19-25. 10.7774/cevr.2016.5.1.19.
Cloning, expression and purification flagellar sheath adhesion of Helicobacter pylori in Escherichia coli host as a vaccination target
- Affiliations
-
- 1Department of Microbiology, Faculty of Biological Sciences, Shahid Beheshti University, Tehran, Iran.
- 2Department of Bacteriology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. mmmobarez@modares.ac.ir
- 3Molecular Genetics, Cancer Research Center, Tehran University of Medical Sciences, Tehran, Iran.
- KMID: 2152693
- DOI: http://doi.org/10.7774/cevr.2016.5.1.19
Abstract
- PURPOSE
Helicobacter pylori is a widely distributed gram-negative bacterium that infects the human stomach and duodenum. HpaA is a H. pylori-specific lipoprotein that has been shown to be an effective protective antigen against H. pylori infection. HpaA of H. pylori as a vaccine antigen is fully competent for stimulation of immune responses. The aim of this project is cloning, expression, and purification flagellar sheath adhesion of H. pylori in Escherichia coli host by fast protein liquid chromatography (FPLC) as a vaccination target.
MATERIALS AND METHODS
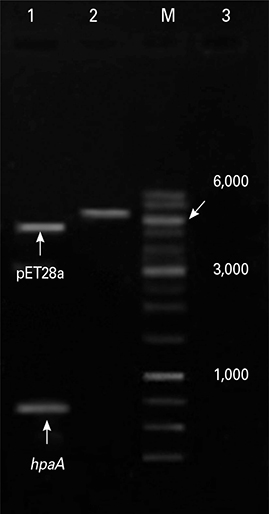
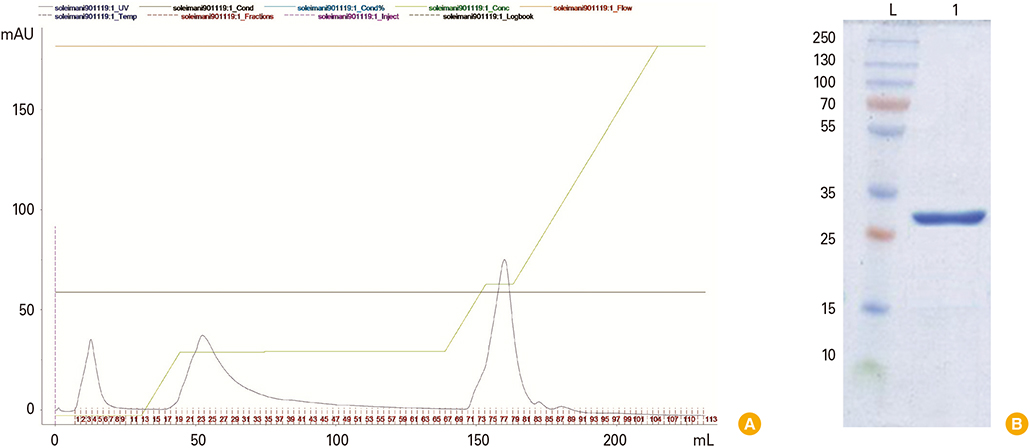
The hpaA gene was inserted into pET28a (+) as cloning and expression vectors respectively. The recombinant plasmid (pET-hpaA) was subjected to sequencing other than polymerase chain reaction (PCR) and digestion analysis. Protein expression was induced by adding 1 mM isopropyl-beta-D-thiogalactoside to cultures of E. coli strain BL21 transformed with pET-hpaA. Protein expression assessed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Protein purification of flagellar sheath adhesion was by FPLC.
RESULTS
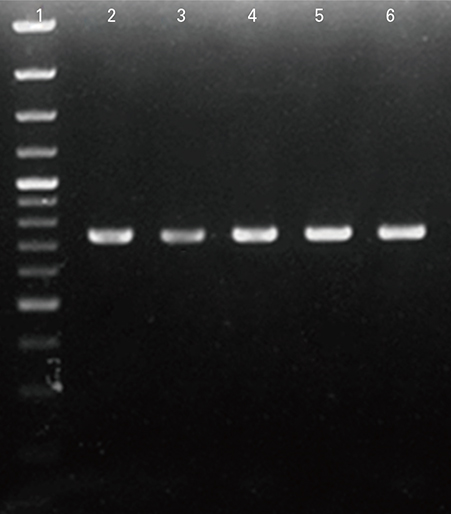
The restriction endonuclease digestion, PCR amplification analysis showed that the hpaA gene of 730 bp was amplified from H. pylori DNA and sequencing analysis of the pET-hpaA confirmed the cloning accuracy and in frame insertion of hpaA fragment. SDS-PAGE analysis showed the expression of an approximately 29,000 Da protein.
CONCLUSION
Sequencing results along with SDS-PAGE analysis confirms the expression of recombinant hpaA in the heterologous E. coli BL21. Conclusion A prokaryotic expression system for hpaA gene was successfully constructed. These results indicate that production of a specific recombinant protein is an alternative and potentially more expeditious strategy for development of H. pylori vaccine.
Keyword
MeSH Terms
-
Chromatography, Liquid
Clone Cells*
Cloning, Organism*
Digestion
DNA
DNA Restriction Enzymes
Duodenum
Electrophoresis, Polyacrylamide Gel
Escherichia coli*
Escherichia*
Helicobacter pylori*
Helicobacter*
Humans
Lipoproteins
Plasmids
Polymerase Chain Reaction
Sodium Dodecyl Sulfate
Stomach
Vaccination*
DNA
DNA Restriction Enzymes
Lipoproteins
Sodium Dodecyl Sulfate
Figure
Reference
-
1. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983; 1:1273–1275.2. Massarrat S, Saberi-Firoozi M, Soleimani A, Himmelmann GW, Hitzges M, Keshavarz H. Peptic ulcer disease, irritable bowel syndrome and constipation in two populations in Iran. Eur J Gastroenterol Hepatol. 1995; 7:427–433.3. Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997; 10:720–741.
Article4. Geis G, Suerbaum S, Forsthoff B, Leying H, Opferkuch W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J Med Microbiol. 1993; 38:371–377.
Article5. Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001; 161:1217–1220.
Article6. Moss SF. Helicobacter pylori and apoptosis: is there consensus. Ital J Gastroenterol Hepatol. 1998; 30:160–161.7. Permin H, Andersen LP. Inflammation, immunity, and vaccines for Helicobacter infection. Helicobacter. 2005; 10:Suppl 1. 21–25.
Article8. Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007; 21:237–259.
Article9. Carlsohn E, Nystrom J, Bolin I, Nilsson CL, Svennerholm AM. HpaA is essential for Helicobacter pylori colonization in mice. Infect Immun. 2006; 74:920–926.
Article10. O'Toole PW, Janzon L, Doig P, Huang J, Kostrzynska M, Trust TJ. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein. J Bacteriol. 1995; 177:6049–6057.11. Lundstrom AM, Blom K, Sundaeus V, Bolin I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb Pathog. 2001; 31:243–253.
Article12. Nystrom J, Svennerholm AM. Oral immunization with HpaA affords therapeutic protective immunity against H. pylori that is reflected by specific mucosal immune responses. Vaccine. 2007; 25:2591–2598.
Article13. Soleimani N, Mohabati Mobarez A, Teymournejad O, Borhani K. Cytotoxicity effect of recombinant outer membrane inflammatory protein (oipA) of Helicobacter pylori on a breast cancer cell line. Modares J Med Sci Pathobiol. 2014; 17:57–66.14. Soleimani N, Mohabati-Mobarez A, Atyabi F, Hasan-Saraf Z, Al Haghighi M. Preparation of chitosan nanoparticles carrying recombinant Helicobacter pylori neutrophil-activating protein. J Mazandaran Univ Med Sci. 2014; 23:134–144.15. Soleimani N, Mobarez AM. Effect of recombinant neutrophil-activating protein (HP-NAP) of Helicobacter pylori on peritoneal macrophages. Iran J Public Health. 2014; 43:234.16. Soleimani N, Mobarez AM, Olia MS, Atyabi F. Synthesis, characterization and effect of the antibacterial activity of chitosan nanoparticles on vancomycin-resistant Enterococcus and other gram negative or gram positive bacteria. Int J Pure Appl Sci Techonol. 2015; 26:14–23.17. Daneshmandi S, Hajimoradi M, Soleimani N, Sattari M. Modulatory effect of Acetobacter xylinum cellulose on peritoneal macrophages. Immunopharmacol Immunotoxicol. 2011; 33:164–168.
Article18. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
Article19. Ueda J, Gosho M, Inui Y, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014; 19:105–110.
Article20. Saad AM, Choudhary A, Bechtold ML. Effect of Helicobacter pylori treatment on gastroesophageal reflux disease (GERD): meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2012; 47:129–135.
Article21. Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998; 115:642–648.
Article22. Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998; 58:4255–4259.23. Salih BA, Abasiyanik MF, Ahmed N. A preliminary study on the genetic profile of cag pathogenicity-island and other virulent gene loci of Helicobacter pylori strains from Turkey. Infect Genet Evol. 2007; 7:509–512.
Article24. Ben Mansour K, Fendri C, Zribi M, et al. Prevalence of Helicobacter pylori vacA, cagA, iceA and oipA genotypes in Tunisian patients. Ann Clin Microbiol Antimicrob. 2010; 9:10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of epidemiological method for the Helicobacter pylori by polymerase chain reaction
- Vaccine Development and Future for Helicobacter pylori
- Development of diagnostic method of helicobacter pylori infection: I. molecular cloning and DNA sequencing of urease
- Purification of the urease of helicobacter pylori and production of monoclonal antibody to the urease of helicobacter pylori
- Perspective of Helicobacter pylori Research: Molecular Pathogenesis of Helicobacter pylori Virulence Factors