Systemic Inflammatory Response Markers and CA-125 Levels in Ovarian Clear Cell Carcinoma: A Two Center Cohort Study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. yssong@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seoungnam, Korea.
- 4Major in Biomodulation, World Class University, Seoul National University, Seoul, Korea.
- KMID: 2152282
- DOI: http://doi.org/10.4143/crt.2014.324
Abstract
- PURPOSE
We compared the predictive and prognostic values of leukocyte differential counts, systemic inflammatory (SIR) markers and cancer antigen 125 (CA-125) levels, and identified the most useful marker in patients with ovarian clear cell carcinoma (OCCC).
MATERIALS AND METHODS
The study included 109 patients with OCCC who did not have any inflammatory conditions except endometriosis, and underwent primary debulking surgery between 1997 and 2012. Leukocyte differential counts (neutrophil, lymphocyte, monocyte, eosinophil, basophil, and platelet), SIR markers including neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), and platelet to lymphocyte ratio (PLR), and CA-125 levels were estimated to select potential markers for clinical outcomes.
RESULTS
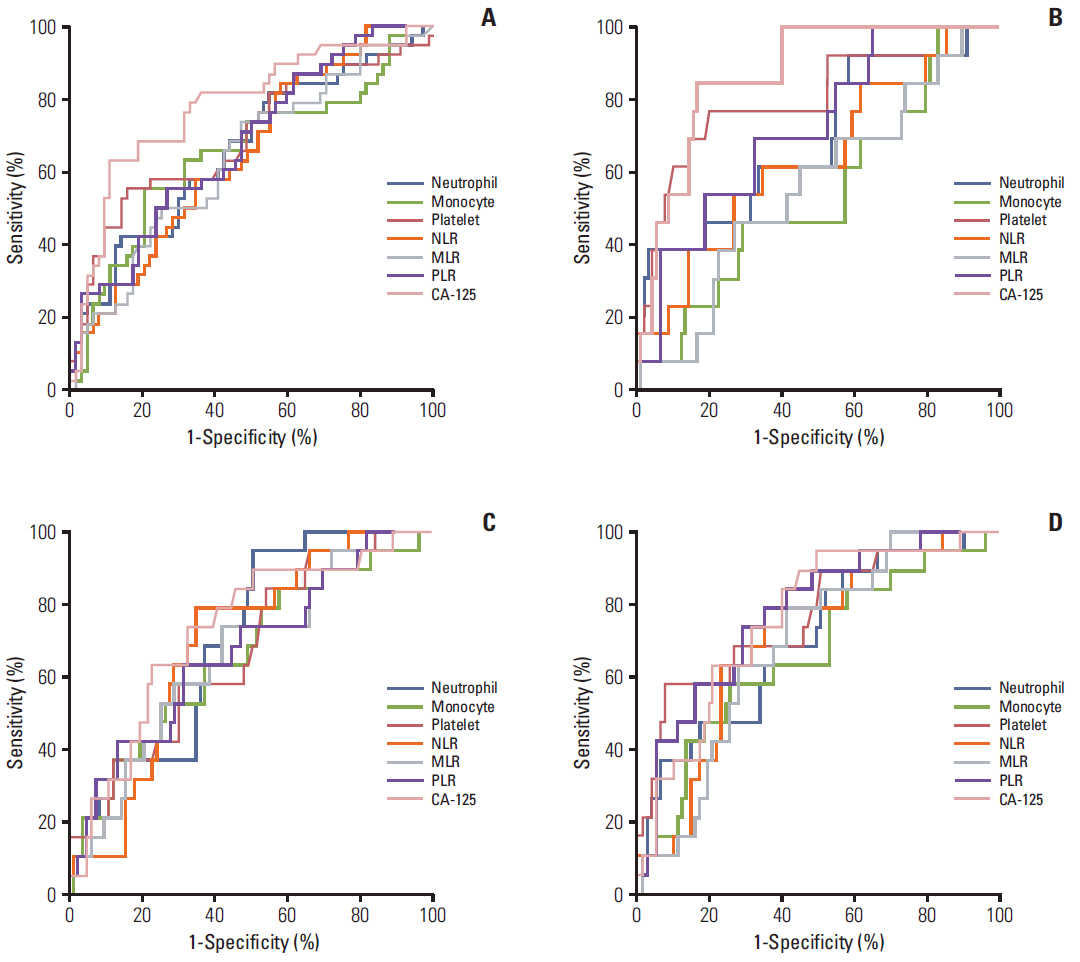
Among potential markers (neutrophil, monocyte, platelet, NLR, MLR, PLR, and CA-125 levels) selected by stepwise comparison, CA-125 levels were best at predicting advanced stage disease, suboptimal debulking and platinum-resistance (cut-off values, > or = 46.5, > or = 11.45, and > or = 66.4 U/mL; accuracies, 69.4%, 78.7%, and 68.5%) while PLR > or = 205.4 predicted non-complete response (CR; accuracy, 71.6%) most accurately. Moreover, PLR < 205.4 was an independent factor for the reduced risk of non-CR (adjusted odds ratio, 0.17; 95% confidence interval [CI], 0.04 to 0.69), and NLR < 2.8 was a favorable factor for improved progression-free survival (PFS; adjusted hazard ratio, 0.49; 95% CI, 0.25 to 0.99) despite lack of a marker for overall survival among the potential markers.
CONCLUSION
CA-125 levels may be the most useful marker for predicting advanced-stage disease. Suboptimal debulking and platinum-resistance, and PLR and NLR may be most effective to predict non-CR and PFS in patients with OCCC.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Prognostic value of neutrophil-to-lymphocyte ratio in early-stage ovarian clear-cell carcinoma
Kosuke Yoshida, Nobuhisa Yoshikawa, Akira Shirakawa, Kaoru Niimi, Shiro Suzuki, Hiroaki Kajiyama, Fumitaka Kikkawa
J Gynecol Oncol. 2019;30(6):. doi: 10.3802/jgo.2019.30.e85.Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis
Yanlin Luo, Hee Seung Kim, Miseon Kim, Maria Lee, Yong Sang Song
J Gynecol Oncol. 2017;28(3):e36. doi: 10.3802/jgo.2017.28.e36.Adipose Stromal Cells from Visceral and Subcutaneous Fat Facilitate Migration of Ovarian Cancer Cells via IL-6/JAK2/STAT3 Pathway
Boyun Kim, Hee Seung Kim, Soochi Kim, Guy Haegeman, Benjamin K. Tsang, Danny N. Dhanasekaran, Yong Sang Song
Cancer Res Treat. 2017;49(2):338-349. doi: 10.4143/crt.2016.175.Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis
Yanlin Luo, Hee Seung Kim, Miseon Kim, Maria Lee, Yong Sang Song
J Gynecol Oncol. 2017;28(3):. doi: 10.3802/jgo.2017.28.e36.
Reference
-
References
1. Suh DH, Kim JW, Kang S, Kim HJ, Lee KH. Major clinical research advances in gynecologic cancer in 2013. J Gynecol Oncol. 2014; 25:236–48.
Article2. Kim JH, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance/anti-Mullerian hormone: A novel treatment for gynecologic tumors. Obstet Gynecol Sci. 2014; 57:343–57.3. Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 2011; 21:419–23.
Article4. Tian C, Markman M, Zaino R, Ozols RF, McGuire WP, Muggia FM, et al. CA-125 change after chemotherapy in prediction of treatment outcome among advanced mucinous and clear cell epithelial ovarian cancers: a Gynecologic Oncology Group study. Cancer. 2009; 115:1395–403.5. Eltabbakh GH, Mount SL, Beatty B, Simmons-Arnold L, Cooper K. Clinical and molecular differences between clear cell and papillary serous ovarian carcinoma. J Surg Oncol. 2006; 93:379–86.
Article6. Shan W, Yang G, Liu J. The inflammatory network: bridging senescent stroma and epithelial tumorigenesis. Front Biosci (Landmark Ed). 2009; 14:4044–57.
Article7. den Ouden M, Ubachs JM, Stoot JE, van Wersch JW. Whole blood cell counts and leucocyte differentials in patients with benign or malignant ovarian tumours. Eur J Obstet Gynecol Reprod Biol. 1997; 72:73–7.
Article8. Kim SI, Kim HS, Kim TH, Suh DH, Kim K, No JH, et al. Impact of underweight after treatment on prognosis of advanced-stage ovarian cancer. J Immunol Res. 2014; 2014:349546.
Article9. Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011; 13:499–503.
Article10. Jilma B, Blann A, Pernerstorfer T, Stohlawetz P, Eichler HG, Vondrovec B, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med. 1999; 159:857–63.11. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357:539–45.
Article12. Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014; 132:542–50.
Article13. Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012; 23:265–73.
Article14. Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009; 58:15–23.
Article15. Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012; 10:33.
Article16. Bishara S, Griffin M, Cargill A, Bali A, Gore ME, Kaye SB, et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2008; 138:71–5.
Article17. Ho CM, Chien TY, Shih BY, Huang SH. Evaluation of complete surgical staging with pelvic and para-aortic lymphadenectomy and paclitaxel plus carboplatin chemotherapy for improvement of survival in stage I ovarian clear cell carcinoma. Gynecol Oncol. 2003; 88:394–9.
Article18. Behbakht K, Randall TC, Benjamin I, Morgan MA, King S, Rubin SC. Clinical characteristics of clear cell carcinoma of the ovary. Gynecol Oncol. 1998; 70:255–8.
Article19. Fader AN, Java J, Krivak TC, Bristow RE, Tergas AI, Bookman MA, et al. The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: a gynecologic Oncology Group study. Gynecol Oncol. 2014; 132:560–5.
Article20. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010; 30:2362–7.21. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999; 59:1295–300.22. Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012; 130:2747–60.
Article23. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011; 105:93–103.
Article24. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106:dju124.
Article25. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014; 23:1204–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Significance of Serum Tumor Markers in Paitents with Ovarian Tumors
- Extremely elevated CA 125 due to an unruptured large endometrioma: A Case Report
- A clinical evaluation of CA 125 antigen values in patients of ovarian cancer
- An Immunohistochemical Study of CA 125, CA 19-9, and CA 15-3 in Ovarian Epithelial Tumors
- A Study on Preoperative Diagnosis in Malignant Ovarian Tumor