Sustaining Blood Lymphocyte Count during Preoperative Chemoradiotherapy as a Predictive Marker for Pathologic Complete Response in Locally Advanced Rectal Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea. okyu.noh@gmail.com
- 2Department of Medicine, The University of Arizona, Tucson, AZ, USA.
- 3BIO5 Institute, The University of Arizona, Tucson, AZ, USA.
- 4Leon Levy Cancer Center, The University of Arizona, Tucson, AZ, USA.
- 5Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 6Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2152280
- DOI: http://doi.org/10.4143/crt.2014.351
Abstract
- PURPOSE
The objective of this study was to explore the relationship between the circulating lymphocyte level during preoperative chemoradiotherapy (CRT) and pathologic complete response (pCR) in locally advanced rectal cancer.
MATERIALS AND METHODS
From May 2010 to May 2013, 52 patients treated with preoperative CRT followed by surgery, were analysed. Patients received conventional fractionated radiotherapy (50-54 Gy) with fluorouracil-based chemotherapy. Surgical resection was performed at 4 to 8 weeks after the completion of preoperative CRT. Absolute blood lymphocyte counts and their relative percentage in total white blood cell counts were obtained from complete blood count tests performed prior to and after 4, 8, and 12 weeks of CRT. We analysed the association between achieving pCR and change in blood lymphocyte level during CRT, as well as clinical parameters.
RESULTS
Among 52 patients, 14 (26.9%) had evidence of pCR. Sustaining the blood lymphocyte count during CRT (lymphocyte count at 4 weeks/baseline lymphocyte count > 0.35; odds ratio, 8.33; p=0.02) and initial carcinoembryonic antigen < 4.4 ng/mL (odds ratio, 6.71; p=0.03) were significantly associated with pCR in multivariate analyses.
CONCLUSION
Sustaining blood lymphocyte count during preoperative CRT was predictive for pCR in rectal cancer. Further studies are warranted to investigate the association between pathologic responses and circulating lymphocyte count with its subpopulation during preoperative CRT.
Keyword
MeSH Terms
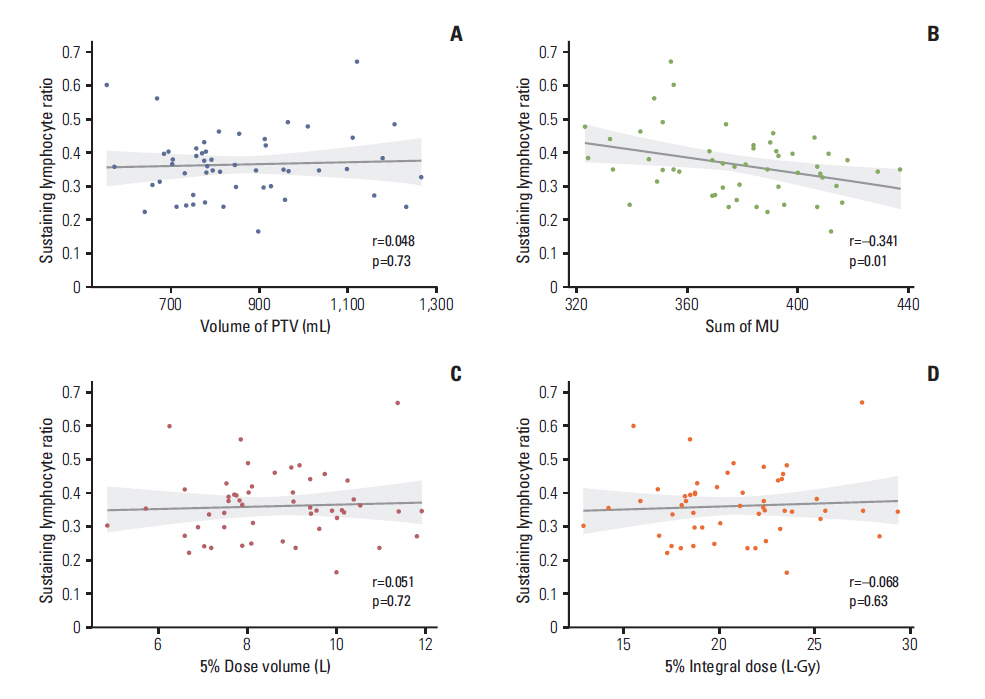
Figure
Cited by 3 articles
-
Prediction of Pathologic Response to Neoadjuvant Chemoradiotherapy in Patients with Esophageal Squamous Cell Carcinoma Incorporating Hematological Biomarkers
Yingjia Wu, Jinbin Chen, Lei Zhao, Qiaoqiao Li, Jinhan Zhu, Hong Yang, Suping Guo, Mian Xi
Cancer Res Treat. 2021;53(1):172-183. doi: 10.4143/crt.2020.594.Predicting stage ypT0–1N0 for nonradical management in patients with middle or low rectal cancer who undergo neoadjuvant chemoradiotherapy: a retrospective cohort study
Jeehye Lee, In Jun Yang, Jung Wook Suh, Hong-min Ahn, Heung-Kwon Oh, Duck-Woo Kim, Young-Hoon Kim, Kyoung Ho Lee, Sung-Bum Kang
Ann Surg Treat Res. 2022;103(1):32-39. doi: 10.4174/astr.2022.103.1.32.The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
Xiaoyan Sun, Yingnan Feng, Bin Zhang, Wuhao Huang, Xiaoliang Zhao, Hua Zhang, Dongsheng Yue, Changli Wang
Cancer Res Treat. 2022;54(4):1017-1029. doi: 10.4143/crt.2021.1007.
Reference
-
References
1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.
Article2. Kong M, Hong SE, Choi WS, Kim SY, Choi J. Preoperative concurrent chemoradiotherapy for locally advanced rectal cancer: treatment outcomes and analysis of prognostic factors. Cancer Res Treat. 2012; 44:104–12.
Article3. Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012; 99:918–28.
Article4. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11:835–44.
Article5. Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010; 252:998–1004.6. Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007; 69:1167–72.
Article7. Restivo A, Zorcolo L, Cocco IM, Manunza R, Margiani C, Marongiu L, et al. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann Surg Oncol. 2013; 20:864–71.
Article8. Rodel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Gunther K, Schick C, et al. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2002; 52:294–303.9. Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009; 74:673–88.
Article10. Choi CH, Kim WD, Lee SJ, Park WY. Clinical predictive factors of pathologic tumor response after preoperative chemoradiotherapy in rectal cancer. Radiat Oncol J. 2012; 30:99–107.
Article11. Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010; 5:47.
Article12. Schmidt MA, Fortsch C, Schmidt M, Rau TT, Fietkau R, Distel LV. Circulating regulatory T cells of cancer patients receiving radiochemotherapy may be useful to individualize cancer treatment. Radiother Oncol. 2012; 104:131–8.
Article13. Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013; 31:183–8.
Article14. Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2013; 31:May. 2. [Epub]. http://dx.doi.org/10.1097/COC.0b013e3182940ff9.
Article15. Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010; 28:105–13.
Article16. Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998; 58:3491–4.17. Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997; 182:318–24.
Article18. Haustermans K, Debucquoy A, Lambrecht M. The ESTRO Breur Lecture 2010: toward a tailored patient approach in rectal cancer. Radiother Oncol. 2011; 100:15–21.
Article19. Lissoni P, Brivio F, Fumagalli L, Messina G, Meregalli S, Porro G, et al. Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. Anticancer Res. 2009; 29:1847–52.20. Schuler PJ, Borger V, Bolke E, Habermehl D, Matuschek C, Wild CA, et al. Dendritic cell generation and CD4+ CD25high FOXP3+ regulatory t cells in human head and neck carcinoma during radio-chemotherapy. Eur J Med Res. 2011; 16:57–62.21. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009; 69:5383–91.
Article22. Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011; 17:5473–80.
Article23. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013; 31:140–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical predictive factors of pathologic tumor response after preoperative chemoradiotherapy in rectal cancer
- Nodal tumor response according to the count of peripheral blood lymphocyte subpopulations during preoperative chemoradiotherapy in locally advanced rectal cancer
- Preoperative Concurrent Chemoradiotherapy for Locally Advanced Rectal Cancer: Treatment Outcomes and Analysis of Prognostic Factors
- How Can We Improve the Tumor Response to Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer?
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer