Cancer Res Treat.
2016 Jan;48(1):153-161. 10.4143/crt.2014.183.
The Role of Plasma Chromogranin A as Assessment of Treatment Response in Non-functioning Gastroenteropancreatic Neuroendocrine Tumors
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. shty1@skku.edu
- 2Gastrointestinal Cancer Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2152271
- DOI: http://doi.org/10.4143/crt.2014.183
Abstract
- PURPOSE
Chromogranin A (CgA) has been considered to be valuable not only in the diagnosis but also in monitoring the disease response to treatment. However, only a few studies have been published on this issue. We purposed to evaluate whether biochemical response using plasma CgA level is reliable in concordance with the clinical response of grade 1-3 nonfunctiong gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
MATERIALS AND METHODS
Between March 2011 and September 2013, a total of 27 cases in 18 patients were analysed, clinically and radiologically while serial CgA tests were also conducted during treatment. Tumor responses were defined by both Response Evaluation Criteria in Solid Tumors (RECIST) criteria ver. 1.1 and biochemical criteria based on the CgA level.
RESULTS
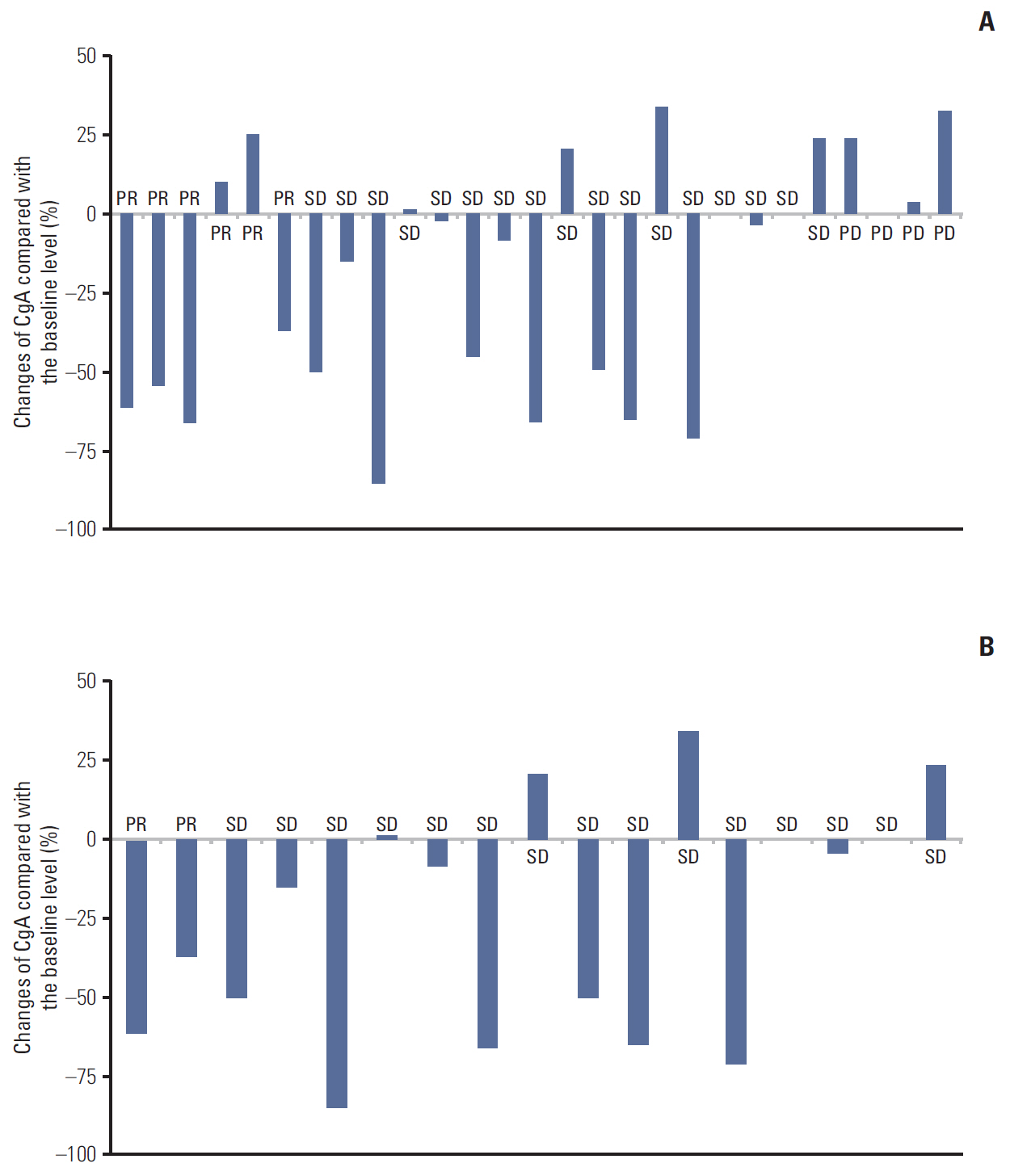
Among the 27 cases analysed, no difference in the basal CgA level was observed with regard to gender, primary tumor site, tumor grade (World Health Organization classification), liver metastasis, number of metastatic site, and line of chemotherapy. The overall response rate (RR) by RECIST criteria ver. 1.1 was six out of the 27 cases (22.2%) and eight out of the 27 cases (29.6%) for biochemical RR. The overall concordance rates of the response based on RECIST and biochemical criteria were 74%. In grades 1 and 2 GEP-NETs (n=17), the concordance rate of the disease control was 94.1%. There was a significant difference for progression-free survival (PFS) between responders and non-responder in accordance to biochemical criteria (35.73 months vs. 5.93 months, p=0.05).
CONCLUSION
This study revealed that changes of the plasma CgA levels were associated with tumour response. Additionally, biochemical response based on serial CgA may be a predictive marker for PFS in GEP-NETs.
MeSH Terms
Figure
Reference
-
References
1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–72.
Article2. Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakk er RV, et al. G astroenteropan creatic ne uroendocrine tumours. Lancet Oncol. 2008; 9:61–72.3. Delaunoit T, Neczyporenko F, Rubin J, Erlichman C, Hobday TJ. Medical management of pancreatic ne uroendocrine tumors. Am J Gastroenterol. 2008; 103:475–83.4. Ramage JK, Davies AH, Ardill J, Bax N, Caplin M, Grossman A, et al. Guidelines for the management of gastroenteropancreati c neuroendocrine (inc luding carcino id) t umours. Gut. 2005; 54 Suppl 4:iv1–16.5. Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neu roendocrin e tumors. J Natl Cancer Inst. 2008; 100:1282–9.6. Warner RR. Enteroendocrine tumors other than carcinoid: a revie w of clinic ally signif icant advances . Gast ro ente ro logy. 2005; 128:1668–84.7. Ganeshan D, Bhosale P, Yang T, Kundra V. Imaging features of carcino id tumors of t h e gastroi n testinal tra ct. AJR Am J Roentgenol. 2013; 201:773–86.8. Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007; 25:1967–73.
Article9. Nobels FR, Kwekkeboom DJ, Bouillon R, Lamberts SW. Chromogranin A: its clinical value as marker of neuroe n docr ine tumours. Eur J Clin Invest. 1998; 28:431–40.10. O'Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986; 314:1145–51.11. Welin S, Stridsberg M, Cunningham J, Granberg D, Skogseid B, Oberg K, et al. Elevated plasma chromogranin A is the first indica tion of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology. 2009; 89:302–7.12. Sondenaa K, Sen J, Heinle F, Fjetland L, Gudlaugsson E, Syversen U. Chromogranin A, a marker of the therapeutic success of resection of neuroendocrine liver metastases: preliminary report. World J Surg. 2004; 28:890–5.13. Baudin E , Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin JC, et al. Neuron-specific enolase and chromogranin A as markers of n euroendocrine tumours. Br J Cancer. 1998; 78:1102–7.14. Jensen KH, Hilsted L, Jensen C, Mynster T, Rehfeld JF, Knigge U. Chromogranin A is a sensitive mark er of progressio n or regressio n in ileo -cecal neuroendocri n e tumo rs . Scand J Gastroenterol. 2013; 48:70–7.15. Walter T, Chardon L, Chopin-laly X, Raverot V, Caffin A G, Chayvialle JA, et al. Is the combination of chromogranin A and pancreatic polypeptide serum determinations of interest in the diagnosis and follow-up of gastro-entero-pancreatic neuroendocrine tumours? Eur J Cancer. 2012; 48:1766–73.16. Nehar D, Lombard-Bohas C, Olivieri S, Claustrat B, Chayvialle JA, Penes MC, et al. Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf). 2004; 60:644–52.
Article17. Chou WC, Hung YS, Hsu JT, Chen JS, Lu CH, Hwang TL, et al. Chromogranin A is a reliable biomarker for gastroenteropancreatic neuroendocrine tumors in an Asian population of patients. Neuroendocrinology. 2012; 95:344–50.
Article18. Bajetta E, Zilembo N, Di Bartolomeo M, Di Leo A, Pilotti S, Bochicchio AM, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer. 1993; 72:3099–105.
Article19. Bajetta E, Ferrari L, Martinetti A, Celio L, Procopio G, Artale S, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999; 86:858–65.
Article20. Abou-Saif A, Gibril F, Ojeaburu JV, Bashir S, Entsuah LK, Asgharian B, et al. Prospective study of the ability of serial measurements of serum chromogranin A and gastrin to detect changes in tumor burden in patients with gastrinomas. Cancer. 2003; 98:249–61.
Article21. Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose KJ, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol. 2008; 6:820–7.
Article22. Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997; 82:2622–8.23. Cimitan M, Buonadonna A, Cannizzaro R, Canzonieri V, Borsatti E, Ruffo R, et al. Somatostatin receptor scintigraphy versus chromogranin A assay in the management of patients with neuroendocrine tumors of different types: clinical role. Ann Oncol. 2003; 14:1135–41.
Article24. Namwongprom S, Wong FC, Tateishi U, Kim EE, Boonyaprapa S. Correlation of chromogranin A levels and somatostatin receptor scintigraphy findings in the evaluation of metastases in carcinoid tumors. Ann Nucl Med. 2008; 22:237–43.
Article25. Zatelli MC, Torta M, Leon A, Ambrosio MR, Gion M, Tomassetti P, et al. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer. 2007; 14:473–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antitumor Effects of Somatostatin Analogs in Gastroenteropancreatic Neuroendocrine Tumors
- Recent Update of Pathology of the Pancreatic Neuroendocrine Tumor
- Gastroenteropancreatic Neuroendocrine Tumor with Hepatic Metastasis Misdiagnosed as Hepatocellular Carcinoma
- Endoscopic Ultrasound in Gastroenteropancreatic Neuroendocrine Tumors
- Clinical Significance of Protein Expression of Cyclooxygenase-2 and Somatostatin Receptors in Gastroenteropancreatic Neuroendocrine Tumors