Cancer Res Treat.
2016 Jan;48(1):54-62. 10.4143/crt.2014.332.
Prediction of Lymph Node Metastasis by Tumor Dimension Versus Tumor Biological Properties in Head and Neck Squamous Cell Carcinomas
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Biomedical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 2Biostatistics and Clinical Epidemiology Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hansin.jeong@gmail.com
- 4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2152260
- DOI: http://doi.org/10.4143/crt.2014.332
Abstract
- PURPOSE
Lymph node metastasis (LNM) is a strong prognostic factor in many solid cancers, including head and neck squamous cell carcinomas (HNSCC), and LNM can be dependent upon primary tumor biology, as well as tumor dimension. Here, we investigate the relative risk of LNM in accordance to tumor dimension and biology in HNSCC subsites.
MATERIALS AND METHODS
Medical data of 295 HNSCC patients who had undergone the initial curative surgery (oral tongue 174, oropharynx 75, hypopharynx 46) were analyzed to identify the significant predictive factor for LNM. Tumor volume and thickness were set as tumor dimensional variables, and biological variables included lymphovascular, perineural invasion, and tumor differentiation. Statistical analyses were conducted to assess the predictability of LNM from variables, and subgroup analyses according to the tumor subsites. In addition, we evaluated the impacts of tumor dimension and biological variables on the treatment outcomes and survival in HNSCC subsites.
RESULTS
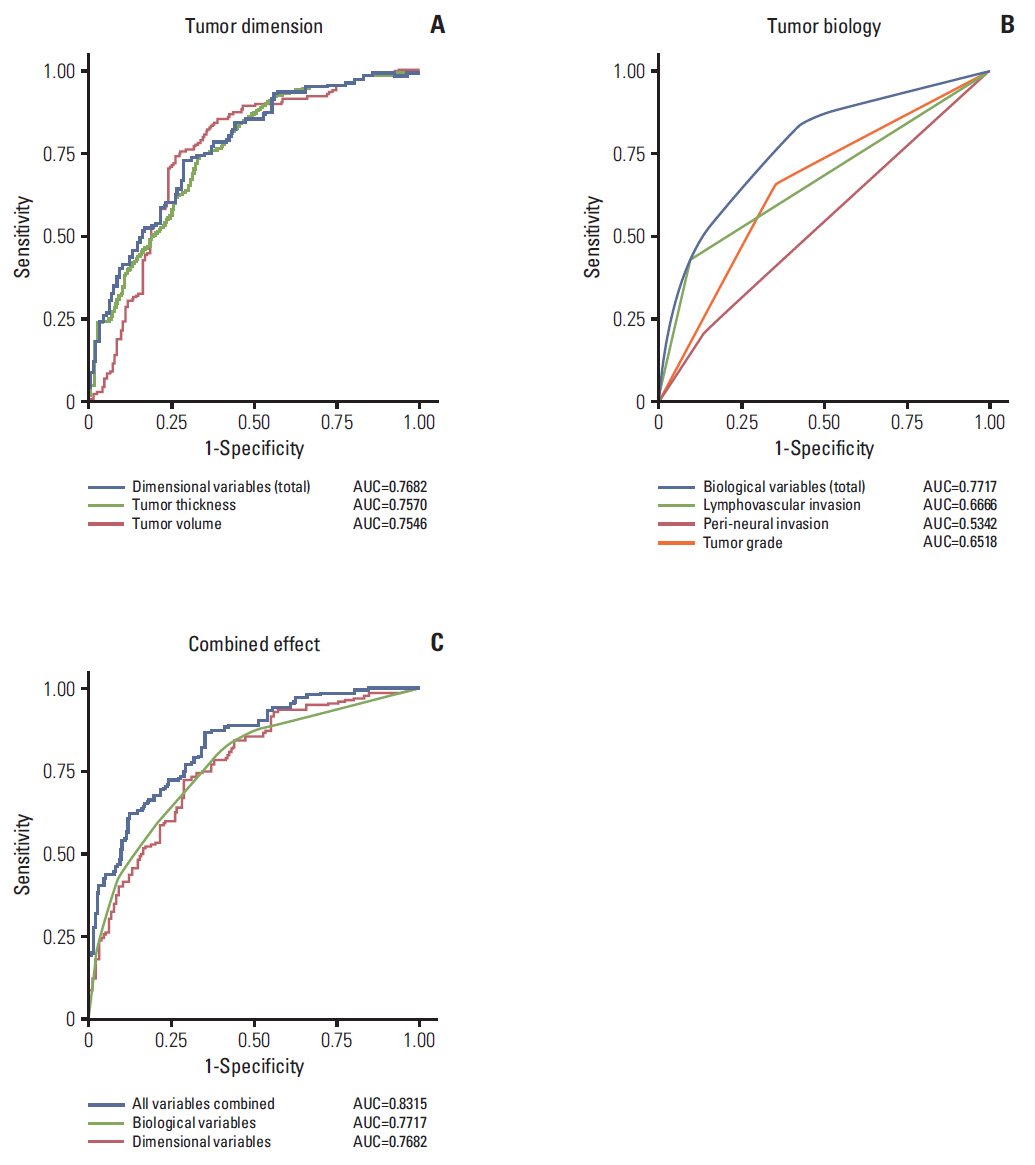
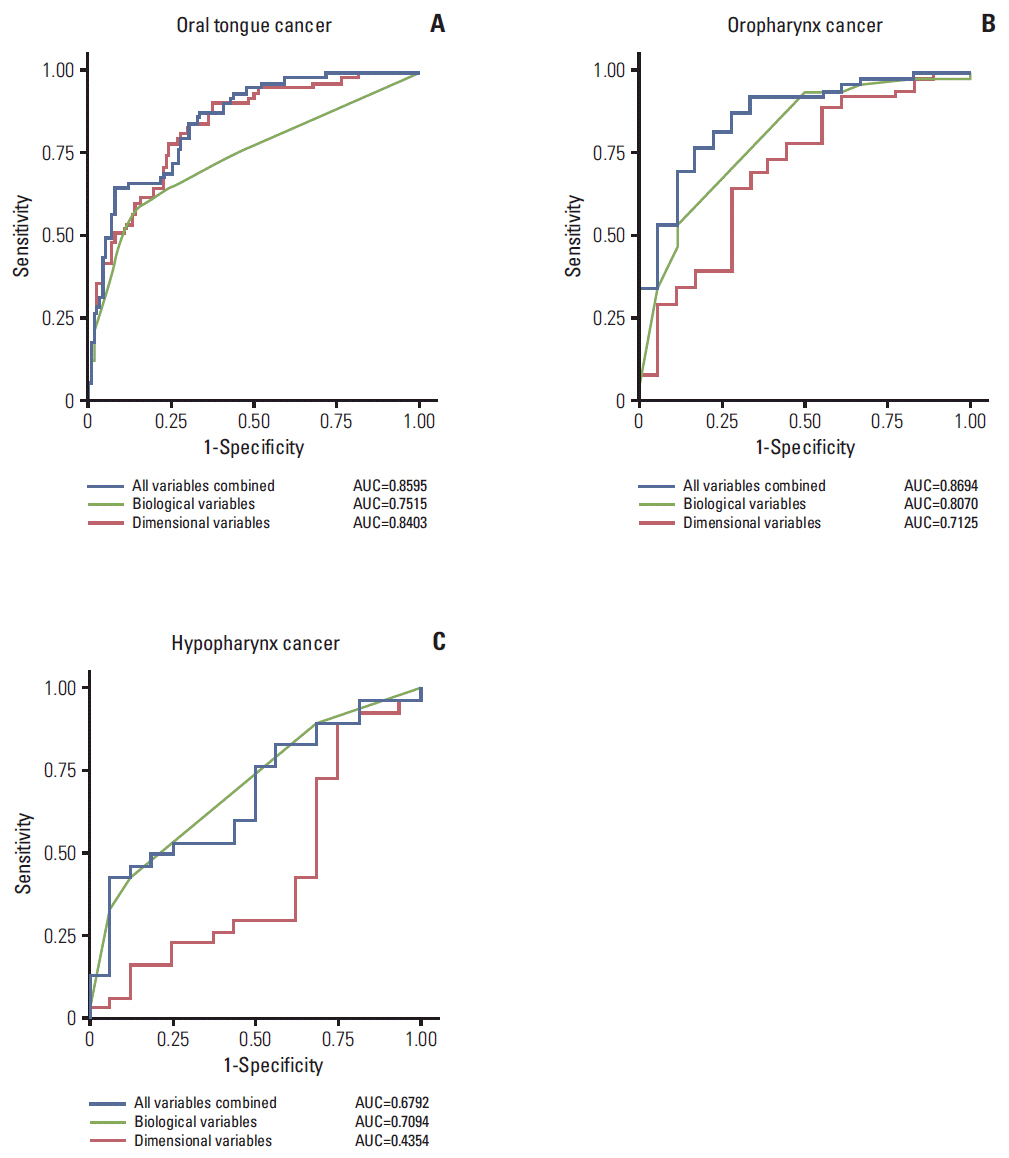
The overall tumor dimension and biological variables had a similar impact on the prediction of LNM in HNSCC (area under the curve, 0.7682 and 0.7717). The prediction sensitivity of LNM in oral tongue cancer was mainly dependent on tumor dimension, while LNM in oroand hypo-pharynx cancers was more influenced by biological factors. Survival analyses also confirmed that biological factor was more powerful in estimating disease-free survival of hypopharyngeal cancer patients, while tumor dimension was more significant in that of oral cancer patients.
CONCLUSION
Tumor dimension and biology have a significant, tumor subsite-dependent impact on the occurrence of LNM and disease-free survival in HNSCC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article2. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004; 4:448–56.
Article3. Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993; 71:452–6.
Article4. Cerezo L, Millan I, Torre A, Aragon G, Otero J. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer: a multivariate study of 492 cases. Cancer. 1992; 69:1224–34.
Article5. Le Tourneau C, Velten M, Jung GM, Bronner G, Flesch H, Borel C. Prognostic indicators for survival in head and neck squamous cell carcinomas: analysis of a series of 621 cases. Head Neck. 2005; 27:801–8.
Article6. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008; 371:1695–709.
Article7. Shah JP, Candela FC, Poddar AK. The patterns of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990; 66:109–13.
Article8. Goerkem M, Braun J, Stoeckli SJ. Evaluation of clinical and histomorphological parameters as potential predictors of occult metastases in sentinel lymph nodes of early squamous cell carcinoma of the oral cavity. Ann Surg Oncol. 2010; 17:527–35.
Article9. Moore C, Flynn MB, Greenberg RA. Evaluation of size in prognosis of oral cancer. Cancer. 1986; 58:158–62.
Article10. Tomifuji M, Imanishi Y, Araki K, Yamashita T, Yamamoto S, Kameyama K, et al. Tumor depth as a predictor of lymph node metastasis of supraglottic and hypopharyngeal cancers. Ann Surg Oncol. 2011; 18:490–6.
Article11. Smeets A, Ryckx A, Belmans A, Wildiers H, Neven P, Floris G, et al. Impact of tumor chronology and tumor biology on lymph node metastasis in breast cancer. Springerplus. 2013; 2:480.
Article12. Chen TC, Wang CP, Ko JY, Yang TL, Hsu CW, Yeh KA, et al. The impact of perineural invasion and/or lymphovascular invasion on the survival of early-stage oral squamous cell carcinoma patients. Ann Surg Oncol. 2013; 20:2388–95.
Article13. Jones HB, Sykes A, Bayman N, Sloan P, Swindell R, Patel M, et al. The impact of lymphovascular invasion on survival in oral carcinoma. Oral Oncol. 2009; 45:10–5.
Article14. Knegjens JL, Hauptmann M, Pameijer FA, Balm AJ, Hoebers FJ, de Bois JA, et al. Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck. 2011; 33:375–82.
Article15. Strongin A, Yovino S, Taylor R, Wolf J, Cullen K, Zimrin A, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012; 82:1823–30.
Article16. Ganly I, Goldstein D, Carlson DL, Patel SG, O'Sullivan B, Lee N, et al. Long-term regional control and survival in patients with "low-risk," early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013; 119:1168–76.17. Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008; 359:1143–54.
Article18. Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011; 11:9–22.
Article19. Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002; 62:7350–6.20. Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010; 11:781–9.
Article21. Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue--clinicopathologic features affecting outcome. Cancer. 2012; 118:101–11.
Article22. Akita H, Doki Y, Yano M, Miyata H, Miyashiro I, Ohigashi H, et al. Effects of neoadjuvant chemotherapy on primary tumor and lymph node metastasis in esophageal squamous cell carcinoma: additive association with prognosis. Dis Esophagus. 2009; 22:291–7.
Article23. Fruhwirth GO, Diocou S, Blower PJ, Ng T, Mullen GE. A whole-body dual-modality radionuclide optical strategy for preclinical imaging of metastasis and heterogeneous treatment response in different microenvironments. J Nucl Med. 2014; 55:686–94.
Article24. Padera TP, Kuo AH, Hoshida T, Liao S, Lobo J, Kozak KR, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol Cancer Ther. 2008; 7:2272–9.
Article25. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005; 27:843.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sentinel Lymph Node Biopsy in the Oral Cavity Cancer
- p53 Mutation Patterns in Primary Head and Neck Squamous Cell Carcinomas and Their Metastatic Neck Nodes
- Expression of E-Cadherin and Matrix Metalloproteinase-2 in Squamous Cell Carcinoma of the Oral Tongue
- A Case of Collision Tumor: Bronchogenic Squamous Cell Carcinoma in Cervical Hodgkin Disease
- Expression of Matrix Metalloproteinase-2,-9 and Extracapsular Spread of Cervical Node Metastasis in the Head and Neck Squamous Cell Carcinoma