J Korean Med Sci.
2014 Nov;29(Suppl 3):S237-S248. 10.3346/jkms.2014.29.S3.S237.
Effect of Endogenous Bone Marrow Derived Stem Cells Induced by AMD-3100 on Expanded Ischemic Flap
- Affiliations
-
- 1Department of Plastic & Reconstructive Surgery, Kangnam Sacred Heart Hospital, Hallym University Medical Center, Halllym University College of Medicine, Seoul, Korea. hiisunj@gmail.com
- 2Department of Plastic and Reconstructive Surgery, Institute for Human Tissue Restoration, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Plastic and Reconstructive Surgery, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- 4Departments of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea.
- 5Departments of Pharmacology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2151419
- DOI: http://doi.org/10.3346/jkms.2014.29.S3.S237
Abstract
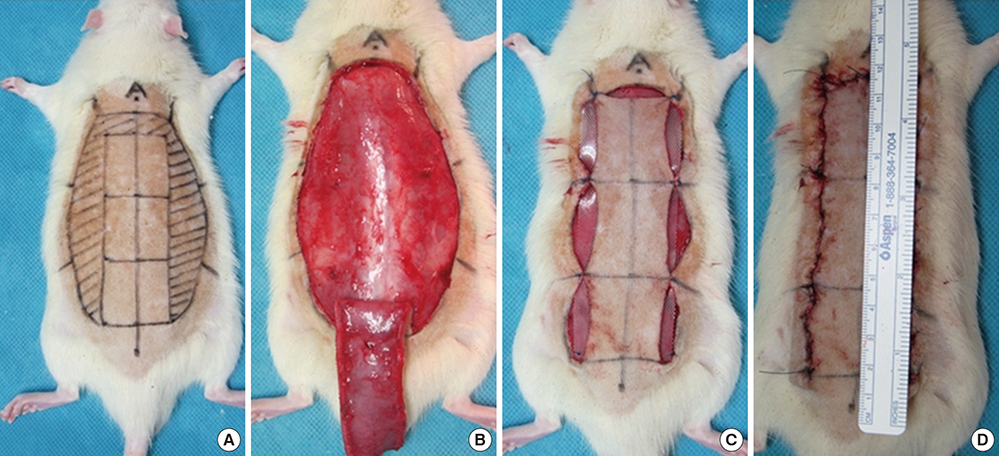
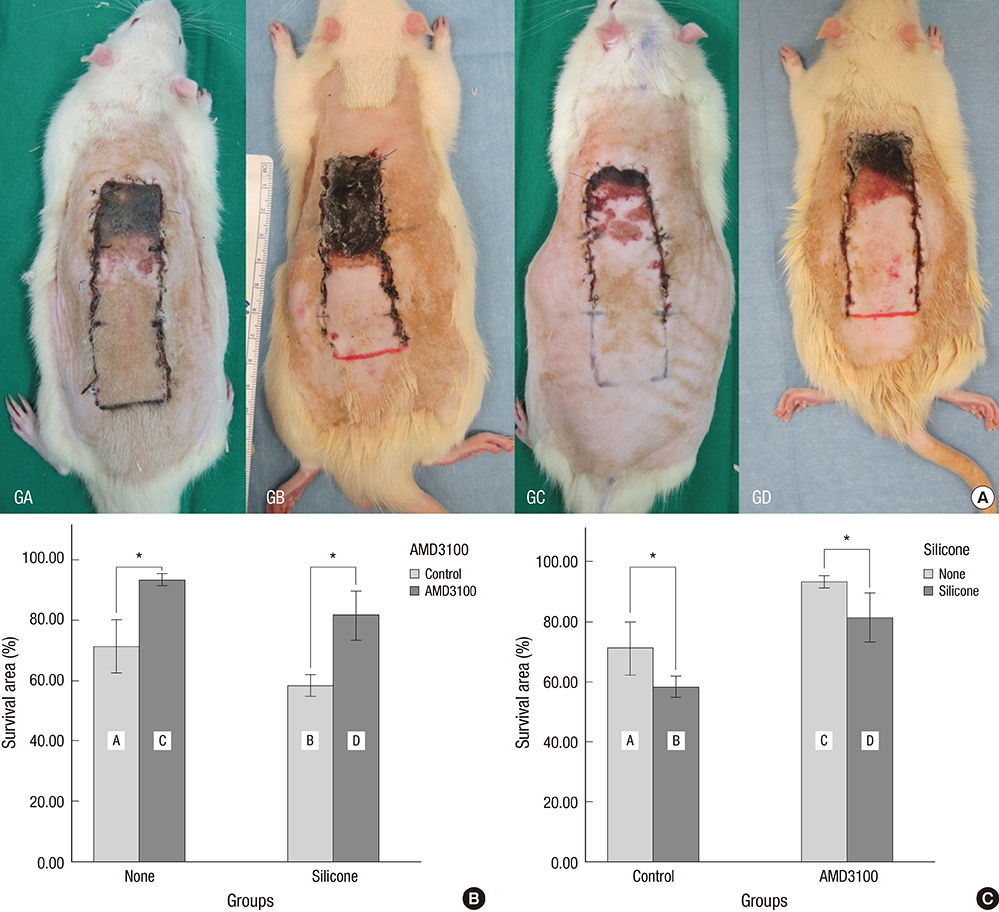
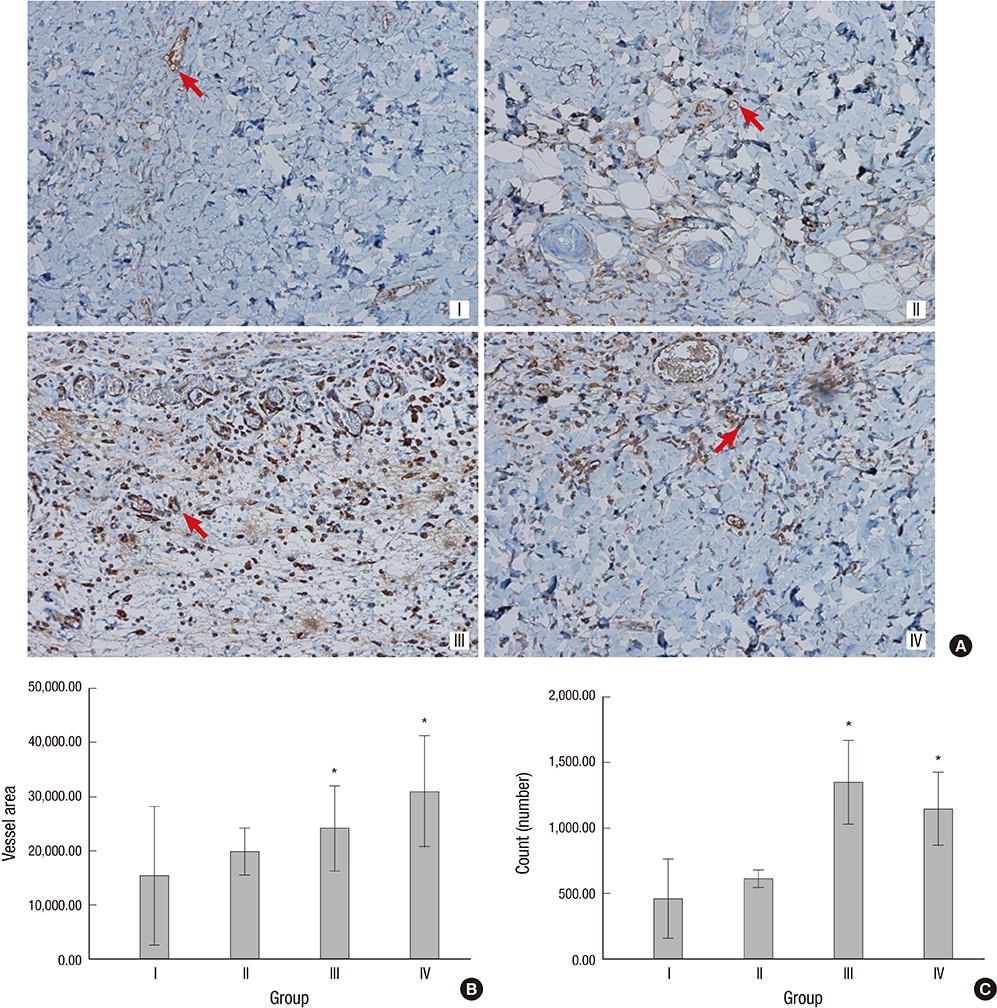
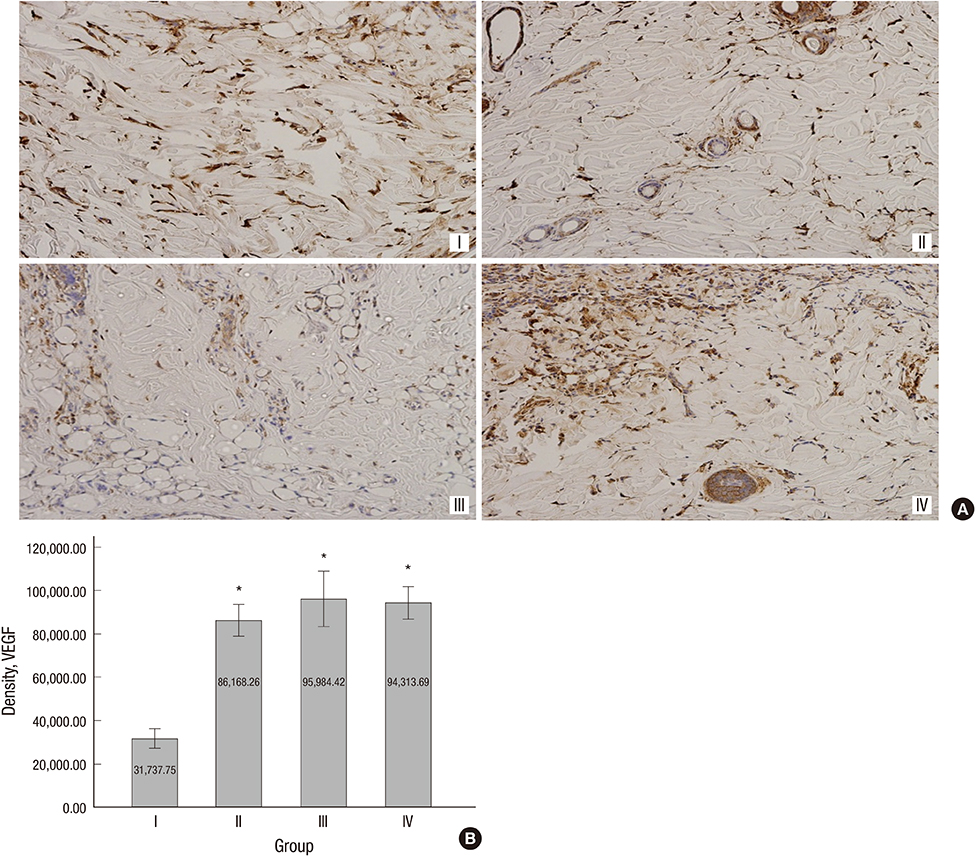
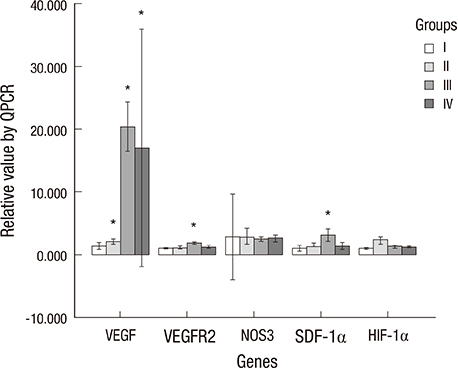
- The purpose of this study was to devise an expanded ischemic flap model and to investigate the role of AMD-3100 (Plerixafor, chemokine receptor 4 inhibitor) in this model by confirming its effect on mobilization of stem cells from the bone marrow. Male Sprague-Dawley rats were used as an animal research model. The mobilization of stem cells from the bone marrow was confirmed in the AMD-3100-treated group. The fractions of endothelial progenitor cells (EPC) and the vascular endothelial growth factor receptor (VEGFR) 2+ cells in the peripheral blood were increased in groups treated with AMD-3100. The expression of vascular endothelial growth factor (VEGF) was increased in response to expansion or AMD injection. The expression of stromal cell derived factor (SDF)-1 and VEGFR2 were increased only in unexpanded flap treated with AMD-3100. Treatment with AMD-3100 increased both the number and area of blood vessels. However, there were no statistically significant differences in the survival area or physiologic microcirculation in rats from the other groups. This endogenous neovascularization induced by AMD-3100 may be a result of the increase in both the area and number of vessels, as well as paracrine augmentation of the expression of VEGF and EPCs. However, the presence of a tissue expander under the flap could block the neovascularization between the flap and the recipient regardless of AMD-3100 treatment and expansion.
Keyword
MeSH Terms
-
Animals
Anti-HIV Agents/pharmacology
Bone Marrow Cells/cytology
Chemokine CXCL12/biosynthesis
Endothelial Progenitor Cells/*cytology
Hematopoietic Stem Cells/*cytology
Heterocyclic Compounds/*pharmacology
Hypoxia-Inducible Factor 1, alpha Subunit/metabolism
Male
Neovascularization, Physiologic
Nitric Oxide Synthase Type III/metabolism
Rats
Rats, Sprague-Dawley
Receptors, CXCR4/antagonists & inhibitors
Surgical Flaps/*blood supply/surgery
Tissue Expansion/*methods
Vascular Endothelial Growth Factor A/biosynthesis
Vascular Endothelial Growth Factor Receptor-2/biosynthesis/metabolism
Anti-HIV Agents
Chemokine CXCL12
Heterocyclic Compounds
Hypoxia-Inducible Factor 1, alpha Subunit
Receptors, CXCR4
Vascular Endothelial Growth Factor A
Nitric Oxide Synthase Type III
Vascular Endothelial Growth Factor Receptor-2
Figure
Reference
-
1. Cho JY, Jang YC, Hur GY, Koh JH, Seo DK, Lee JW, Choi JK. One stage reconstruction of skull exposed by burn injury using a tissue expansion technique. Arch Plast Surg. 2012; 39:118–123.2. Gur E, Zuker RM. Complex facial nevi: a surgical algorithm. Plast Reconstr Surg. 2000; 106:25–35.3. Zuker RM, Filler RM, Lalla R. Intra-abdominal tissue expansion: an adjunct in the separation of conjoined twins. J Pediatr Surg. 1986; 21:1198–1200.4. Sasaki GH, Pang CY. Pathophysiology of skin flaps raised on expanded pig skin. Plast Reconstr Surg. 1984; 74:59–67.5. Cherry GW, Austad E, Pasyk K, McClatchey K, Rohrich RJ. Increased survival and vascularity of random-pattern skin flaps elevated in controlled, expanded skin. Plast Reconstr Surg. 1983; 72:680–687.6. Lantieri LA, Martin-Garcia N, Wechsler J, Mitrofanoff M, Raulo Y, Baruch JP. Vascular endothelial growth factor expression in expanded tissue: a possible mechanism of angiogenesis in tissue expansion. Plast Reconstr Surg. 1998; 101:392–398.7. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004; 10:858–864.8. Takei T, Mills I, Arai K, Sumpio BE. Molecular basis for tissue expansion: clinical implications for the surgeon. Plast Reconstr Surg. 1998; 102:247–258.9. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.10. Sung HM, Suh IS, Lee HB, Tak KS, Moon KM, Jung MS. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 2012; 39:51–54.11. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.12. Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999; 103:1231–1236.13. Kolonin MG, Simmons PJ. Combinatorial stem cell mobilization. Nat Biotechnol. 2009; 27:252–253.14. Brave M, Farrell A, Ching Lin S, Ocheltree T, Pope Miksinski S, Lee SL, Saber H, Fourie J, Tornoe C, Booth B, et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010; 78:282–288.15. Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009; 4:62–72.16. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29:e45.17. De Filippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002; 109:2450–2462.18. Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005; 105:1068–1077.19. Park S, Tepper OM, Galiano RD, Capla JM, Baharestani S, Kleinman ME, Pelo CR, Levine JP, Gurtner GC. Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg. 2004; 113:284–293.20. Zan T, Li Q, Dong J, Zheng S, Xie Y, Yu D, Zheng D, Gu B. Transplanted endothelial progenitor cells increase neo-vascularisation of rat pre-fabricated flaps. J Plast Reconstr Aesthet Surg. 2010; 63:474–481.21. Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011; 29:1650–1655.22. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999; 5:434–438.23. Borgquist O, Ingemansson R, Malmsjö M. Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from -10 to -175 mmHg. Plast Reconstr Surg. 2010; 125:502–509.24. Djedovic G, Kronberger P, Pierer G, Rieger UM. Technical note on vacuum assisted closure-basket fixation of scrotal skin grafts. Arch Plast Surg. 2013; 40:641–642.25. Park CW, Kim YH, Hwang KT, Kim JT. Reconstruction of a severely crushed leg with interpositional vessel grafts and latissimus dorsi flap. Arch Plast Surg. 2012; 39:417–421.26. Yang M, Li Q, Sheng L, Li H, Weng R, Zan T. Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Ann Surg. 2011; 253:202–209.27. Shrader CD, Ressetar HG, Luo J, Cilento EV, Reilly FD. Acute stretch promotes endothelial cell proliferation in wounded healing mouse skin. Arch Dermatol Res. 2008; 300:495–504.28. Chenwang D, Shiwei B, Dashan Y, Qiang L, Bin C, Muxin Z, Pengcheng L, Senkai L. Application of botulinum toxin type A in myocutaneous flap expansion. Plast Reconstr Surg. 2009; 124:1450–1457.29. Tang Y, Luan J, Zhang X. Accelerating tissue expansion by application of topical papaverine cream. Plast Reconstr Surg. 2004; 114:1166–1169.30. Vinnik CA, Jacob SW. Dimethylsulfoxide (DMSO) for human single-stage intraoperative tissue expansion and circulatory enhancement. Aesthetic Plast Surg. 1991; 15:327–337.31. Copcu E, Sivrioglu N, Sisman N, Aktas A, Oztan Y. Enhancement of tissue expansion by calcium channel blocker: a preliminary study. World J Surg Oncol. 2003; 1:19.32. Zhu X, Hall D, Ridenour G, Boo S, Jennings T, Hochberg J, Cilento E, Reilly FD. A mouse model for studying rapid intraoperative methods of skin closure and wound healing. Med Sci Monit. 2003; 9:BR109–BR115.33. De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004; 104:3472–3482.34. Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, Ito A, Misener S, Tanaka T, Klyachko E, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010; 107:11008–11013.35. Erba P, Miele LF, Adini A, Ackermann M, Lamarche JM, Orgill BD, D'Amato RJ, Konerding MA, Mentzer SJ, Orgill DP. A morphometric study of mechanotransductively induced dermal neovascularization. Plast Reconstr Surg. 2011; 128:288e–299e.36. Pompilio G, Capogrossi MC, Pesce M, Alamanni F, DiCampli C, Achilli F, Germani A, Biglioli P. Endothelial progenitor cells and cardiovascular homeostasis: clinical implications. Int J Cardiol. 2009; 131:156–167.37. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219.38. Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, Ogihara T, Kaneda Y, Kohno M, Morishita R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003; 108:2250–2257.39. Amano K, Matsubara H, Iba O, Okigaki M, Fujiyama S, Imada T, Kojima H, Nozawa Y, Kawashima S, Yokoyama M, et al. Enhancement of ischemia-induced angiogenesis by eNOS overexpression. Hypertension. 2003; 41:156–162.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Autologous Bone Marrow Derived Stem Cells for the Treatment of Multiple Sclerosis
- Endogenous Stem Cells in Homeostasis and Aging
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Effect of Nitric Oxide on the Viability of Bone Marrow - Derived Cultured Mast Cells