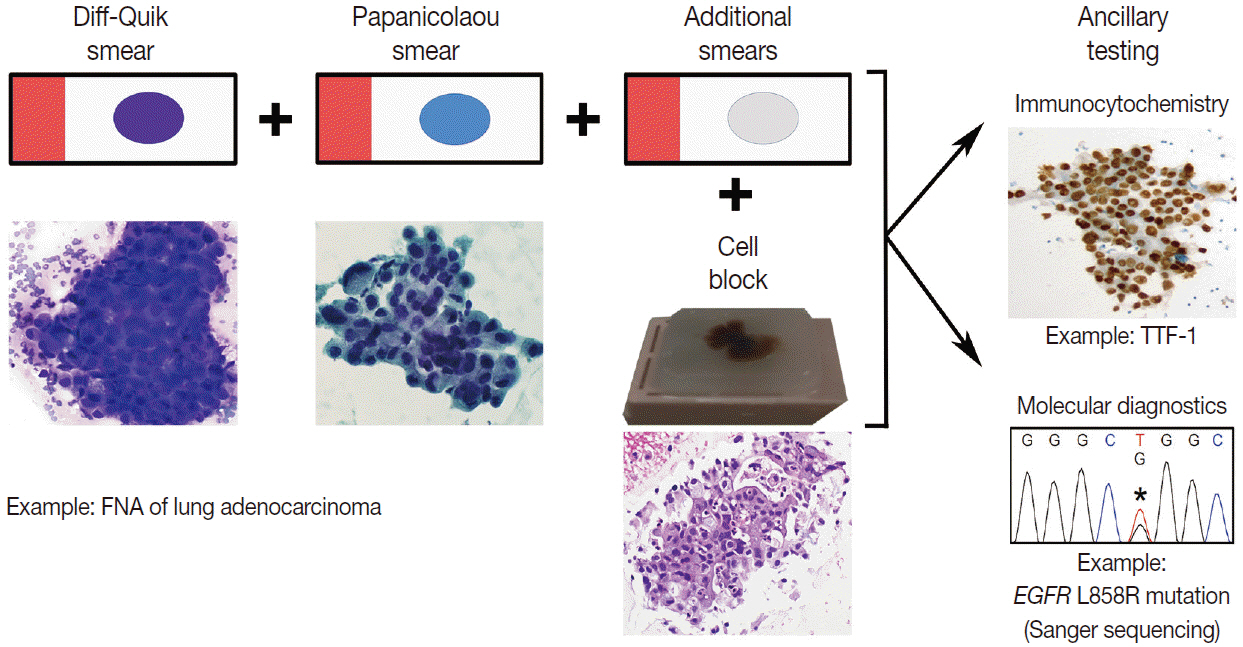
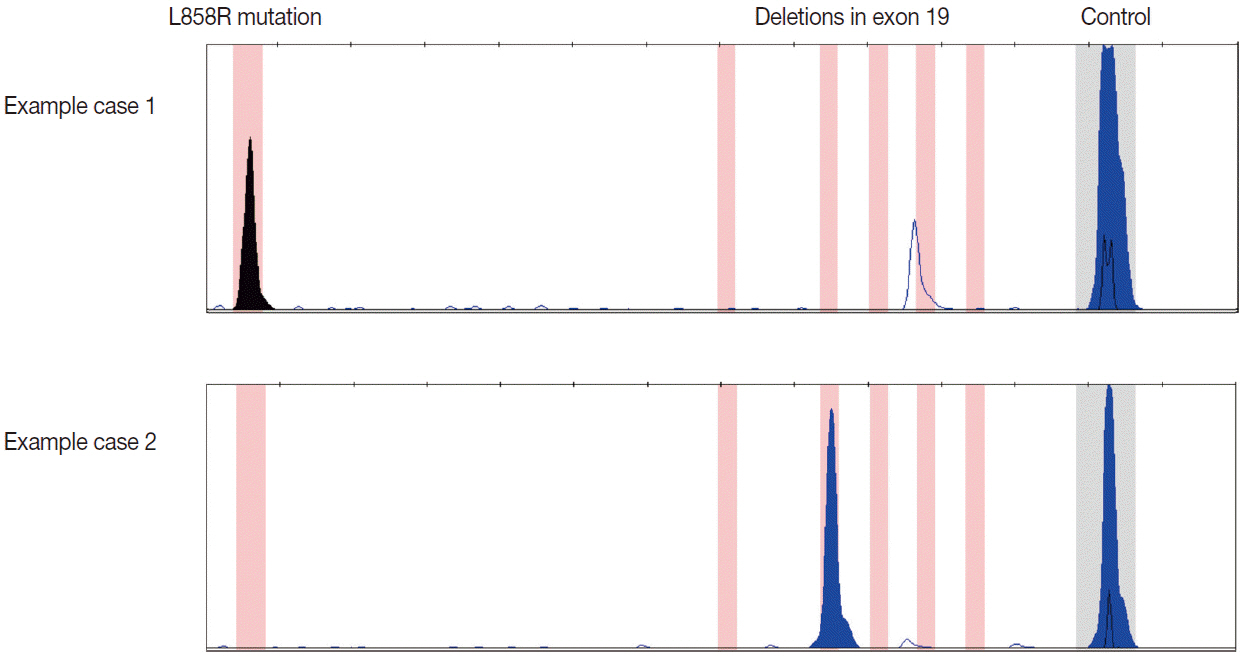
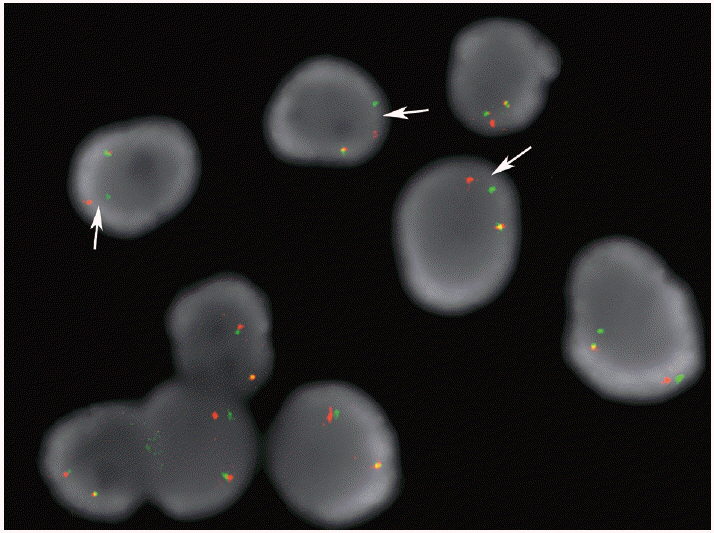
J Pathol Transl Med.
2015 Jul;49(4):300-309. 10.4132/jptm.2015.06.16.
The Utilization of Cytologic Fine-Needle Aspirates of Lung Cancer for Molecular Diagnostic Testing
- Affiliations
-
- 1Department of Pathology, University of Michigan Health System, Ann Arbor, MI, USA. mikro@med.umich.edu
- KMID: 2151139
- DOI: http://doi.org/10.4132/jptm.2015.06.16
Abstract
- In this era of precision medicine, our understanding and knowledge of the molecular landscape associated with lung cancer pathogenesis continues to evolve. This information is being increasingly exploited to treat advanced stage lung cancer patients with tailored, targeted therapy. During the management of these patients, minimally invasive procedures to obtain samples for tissue diagnoses are desirable. Cytologic fine-needle aspirates are often utilized for this purpose and are important not only for rendering diagnoses to subtype patients' lung cancers, but also for ascertaining molecular diagnostic information for treatment purposes. Thus, cytologic fine-needle aspirates must be utilized and triaged judiciously to achieve both objectives. In this review, strategies in utilizing fine-needle aspirates will be discussed in the context of our current understanding of the clinically actionable molecular aberrations underlying non-small cell lung cancer and the molecular assays applied to these samples in order to obtain treatment-relevant molecular diagnostic information.
MeSH Terms
Figure
Cited by 1 articles
-
Biomarker testing of cytology specimens in personalized medicine for lung cancer patients
Hyojin Kim, Jin-Haeng Chung
J Pathol Transl Med. 2022;56(6):326-333. doi: 10.4132/jptm.2022.10.17.
Reference
-
1. Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol. 2012; 138:332–46.2. Allegrini S, Antona J, Mezzapelle R, et al. Epidermal growth factor receptor gene analysis with a highly sensitive molecular assay in routine cytologic specimens of lung adenocarcinoma. Am J Clin Pathol. 2012; 138:377–81.
Article3. Dacic S. Molecular genetic testing for lung adenocarcinomas: a practical approach to clinically relevant mutations and translocations. J Clin Pathol. 2013; 66:870–4.
Article4. Knoepp SM, Roh MH. Ancillary techniques on direct-smear aspirate slides: a significant evolution for cytopathology techniques. Cancer Cytopathol. 2013; 121:120–8.5. Roh MH. Triage of cytologic direct smears for ancillary studies: a case-based illustration and review. Arch Pathol Lab Med. 2013; 137:1185–90.
Article6. Bellevicine C, Malapelle U, Vigliar E, de Luca C, Troncone G. Epidermal growth factor receptor test performed on liquid-based cytology lung samples: experience of an academic referral center. Acta Cytol. 2014; 58:589–94.
Article7. Bellevicine C, Malapelle U, de Luca C, Iaccarino A, Troncone G. EGFR analysis: current evidence and future directions. Diagn Cytopathol. 2014; 42:984–92.
Article8. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011; 6:49–69.9. Betz BL, Roh MH, Weigelin HC, et al. The application of molecular diagnostic studies interrogating EGFR and KRAS mutations to stained cytologic smears of lung carcinoma. Am J Clin Pathol. 2011; 136:564–71.10. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. 2013; 15:415–53.
Article11. Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–8.
Article12. Katayama T, Matsuo K, Kosaka T, Sueda T, Yatabe Y, Mitsudomi T. Effect of gefitinib on the survival of patients with recurrence of lung adenocarcinoma after surgery: a retrospective case-matching cohort study. Surg Oncol. 2010; 19:e144–9.
Article13. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.14. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–8.
Article15. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article16. Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic nonsmall-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012; 13:466–75.
Article17. Aisner DL, Sams SB. The role of cytology specimens in molecular testing of solid tumors: techniques, limitations, and opportunities. Diagn Cytopathol. 2012; 40:511–24.
Article18. Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013; 66:79–89.19. Hookim K, Roh MH, Willman J, et al. Application of immunocytochemistry and BRAF mutational analysis to direct smears of metastatic melanoma. Cancer Cytopathol. 2012; 120:52–61.
Article20. Chowdhuri SR, Xi L, Pham TH, et al. EGFR and KRAS mutation analysis in cytologic samples of lung adenocarcinoma enabled by laser capture microdissection. Mod Pathol. 2012; 25:548–55.21. da Cunha Santos G, Saieg MA, Geddie W, Leighl N. EGFR gene status in cytological samples of nonsmall cell lung carcinoma: controversies and opportunities. Cancer Cytopathol. 2011; 119:80–91.22. Bernacki KD, Betz BL, Weigelin HC, et al. Molecular diagnostics of melanoma fine-needle aspirates: a cytology-histology correlation study. Am J Clin Pathol. 2012; 138:670–7.23. Killian JK, Walker RL, Suuriniemi M, et al. Archival fine-needle aspiration cytopathology (FNAC) samples: untapped resource for clinical molecular profiling. J Mol Diagn. 2010; 12:739–45.24. Dejmek A, Zendehrokh N, Tomaszewska M, Edsjö A. Preparation of DNA from cytological material: effects of fixation, staining, and mounting medium on DNA yield and quality. Cancer Cytopathol. 2013; 121:344–53.25. Billah S, Stewart J, Staerkel G, Chen S, Gong Y, Guo M. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol. 2011; 119:111–7.26. Boldrini L, Gisfredi S, Ursino S, et al. Mutational analysis in cytological specimens of advanced lung adenocarcinoma: a sensitive method for molecular diagnosis. J Thorac Oncol. 2007; 2:1086–90.
Article27. Bozzetti C, Negri FV, Azzoni C, et al. Epidermal growth factor receptor and Kras gene expression: reliability of mutational analysis on cytological samples. Diagn Cytopathol. 2013; 41:595–8.28. Bruno P, Mariotta S, Ricci A, et al. Reliability of direct sequencing of EGFR: comparison between cytological and histological samples from the same patient. Anticancer Res. 2011; 31:4207–10.29. Khode R, Larsen DA, Culbreath BC, et al. Comparative study of epidermal growth factor receptor mutation analysis on cytology smears and surgical pathology specimens from primary and metastatic lung carcinomas. Cancer Cytopathol. 2013; 121:361–9.
Article30. Lozano MD, Zulueta JJ, Echeveste JI, et al. Assessment of epidermal growth factor receptor and K-ras mutation status in cytological stained smears of non-small cell lung cancer patients: correlation with clinical outcomes. Oncologist. 2011; 16:877–85.
Article31. Mitiushkina NV, Iyevleva AG, Poltoratskiy AN, et al. Detection of EGFR mutations and EML4-ALK rearrangements in lung adenocarcinomas using archived cytological slides. Cancer Cytopathol. 2013; 121:370–6.32. Nomoto K, Tsuta K, Takano T, et al. Detection of EGFR mutations in archived cytologic specimens of non-small cell lung cancer using high-resolution melting analysis. Am J Clin Pathol. 2006; 126:608–15.33. Pang B, Dettmer M, Ong CW, et al. The positive impact of cytological specimens for EGFR mutation testing in non-small cell lung cancer: a single South East Asian laboratory’s analysis of 670 cases. Cytopathology. 2012; 23:229–36.
Article34. Smith GD, Chadwick BE, Willmore-Payne C, Bentz JS. Detection of epidermal growth factor receptor gene mutations in cytology specimens from patients with non-small cell lung cancer utilising high-resolution melting amplicon analysis. J Clin Pathol. 2008; 61:487–93.
Article35. Sun PL, Jin Y, Kim H, Lee CT, Jheon S, Chung JH. High concordance of EGFR mutation status between histologic and corresponding cytologic specimens of lung adenocarcinomas. Cancer Cytopathol. 2013; 121:311–9.36. van Eijk R, Licht J, Schrumpf M, et al. Rapid KRAS, EGFR, BRAF and PIK3CA mutation analysis of fine needle aspirates from nonsmall-cell lung cancer using allele-specific qPCR. PLoS One. 2011; 6:e17791.37. Lee YS, Jin GY, Han YM, Chung MJ, Park HS. Computed tomography-guided transthoracic needle aspiration biopsy of intrapulmonary lesions: utility of a liquid-based cytopreparatory technique. Acta Cytol. 2008; 52:665–70.38. Malapelle U, de Rosa N, Bellevicine C, et al. EGFR mutations detection on liquid-based cytology: is microscopy still necessary? J Clin Pathol. 2012; 65:561–4.39. Malapelle U, de Rosa N, Rocco D, et al. EGFR and KRAS mutations detection on lung cancer liquid-based cytology: a pilot study. J Clin Pathol. 2012; 65:87–91.40. Reynolds JP, Tubbs RR, Minca EC, et al. EGFR mutational genotyping of liquid based cytology samples obtained via fine needle aspiration (FNA) at endobronchial ultrasound of non-small cell lung cancer (NSCLC). Lung Cancer. 2014; 86:158–63.41. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007; 7:169–81.
Article42. Ambrosini-Spaltro A, Campanini N, Bortesi B, et al. EGFR mutation-specific antibodies in pulmonary adenocarcinoma: a comparison with DNA direct sequencing. Appl Immunohistochem Mol Morphol. 2012; 20:356–62.43. Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn. 2010; 12:169–76.44. Hasanovic A, Ang D, Moreira AL, Zakowski MF. Use of mutation specific antibodies to detect EGFR status in small biopsy and cytology specimens of lung adenocarcinoma. Lung Cancer. 2012; 77:299–305.
Article45. Kato Y, Peled N, Wynes MW, et al. Novel epidermal growth factor receptor mutation-specific antibodies for non-small cell lung cancer: immunohistochemistry as a possible screening method for epidermal growth factor receptor mutations. J Thorac Oncol. 2010; 5:1551–8.
Article46. Kitamura A, Hosoda W, Sasaki E, Mitsudomi T, Yatabe Y. Immunohistochemical detection of EGFR mutation using mutation-specific antibodies in lung cancer. Clin Cancer Res. 2010; 16:3349–55.
Article47. Kozu Y, Tsuta K, Kohno T, et al. The usefulness of mutation-specific antibodies in detecting epidermal growth factor receptor mutations and in predicting response to tyrosine kinase inhibitor therapy in lung adenocarcinoma. Lung Cancer. 2011; 73:45–50.
Article48. Yu J, Kane S, Wu J, et al. Mutation-specific antibodies for the detection of EGFR mutations in non-small-cell lung cancer. Clin Cancer Res. 2009; 15:3023–8.49. Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009; 15:5216–23.
Article50. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448:561–6.51. Thunnissen E, Bubendorf L, Dietel M, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch. 2012; 461:245–57.52. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012; 13:1011–9.
Article53. Betz BL, Dixon CA, Weigelin HC, Knoepp SM, Roh MH. The use of stained cytologic direct smears for ALK gene rearrangement analysis of lung adenocarcinoma. Cancer Cytopathol. 2013; 121:489–99.54. Minca EC, Lanigan CP, Reynolds JP, et al. ALK status testing in non-small-cell lung carcinoma by FISH on ThinPrep slides with cytology material. J Thorac Oncol. 2014; 9:464–8.
Article55. Neat MJ, Foot NJ, Hicks A, et al. ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer. Cytopathology. 2013; 24:356–64.56. Proietti A, Alì G, Pelliccioni S, et al. Anaplastic lymphoma kinase gene rearrangements in cytological samples of non-small cell lung cancer: comparison with histological assessment. Cancer Cytopathol. 2014; 122:445–53.
Article57. Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010; 16:1561–71.58. Savic S, Bode B, Diebold J, et al. Detection of ALK-positive nonsmall-cell lung cancers on cytological specimens: high accuracy of immunocytochemistry with the 5A4 clone. J Thorac Oncol. 2013; 8:1004–11.
Article59. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012; 30:863–70.60. Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011; 29:2046–51.61. Luk PP, Yu B, Ng CC, et al. BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res. 2015; 4:142–8.62. Gailey MP, Stence AA, Jensen CS, Ma D. Multiplatform comparison of molecular oncology tests performed on cytology specimens and formalin-fixed, paraffin-embedded tissue. Cancer Cytopathol. 2015; 123:30–9.
Article63. Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia. 2015; 17:385–99.
Article64. Kanagal-Shamanna R, Portier BP, Singh RR, et al. Next-generation sequencing-based multi-gene mutation profiling of solid tumors using fine needle aspiration samples: promises and challenges for routine clinical diagnostics. Mod Pathol. 2014; 27:314–27.
Article65. Karnes HE, Duncavage EJ, Bernadt CT. Targeted next-generation sequencing using fine-needle aspirates from adenocarcinomas of the lung. Cancer Cytopathol. 2014; 122:104–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytologic Classification of Fibrocystic Disease of the Breast: A Proposal for Use of Cytologic Criteria Grading System
- Pulmonary Nocardiosis Diagnosed by Fine Needle Aspiration: A Case Report
- A Cytologic Study of Fine Needle Aspiration Biopsy of Salivary Gland Diseases
- The diagnostic value of ultrasound-guided fine-needle aspiration biopsy in breast masses
- Fine Needle Aspiration Cytology of Myxoid Liposarcoma of the Mediastinum