Immune Netw.
2015 Feb;15(1):27-36. 10.4110/in.2015.15.1.27.
Protection Against Salmonella Typhimurium, Salmonella Gallinarum, and Salmonella Enteritidis Infection in Layer Chickens Conferred by a Live Attenuated Salmonella Typhimurium Strain
- Affiliations
-
- 1College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Jeonju 561-756, Korea. johnhlee@chonbuk.ac.kr
- KMID: 2150827
- DOI: http://doi.org/10.4110/in.2015.15.1.27
Abstract
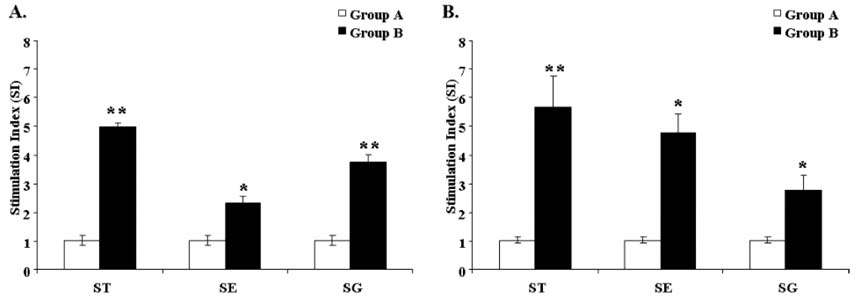
- In the present study, we investigated the protection conferred by a live attenuated Salmonella enterica serovar Typhimurium (ST) strain against Salmonella Typhimurium, Salmonella Gallinarum (SG), and Salmonella Enteritidis (SE) infection in layer chickens. Birds were orally primed with the attenuated ST strain at 7 days of age and then boosted at 4 weeks post prime immunization (PPI). Sequential monitoring of plasma IgG and mucosal secretory IgA (sIgA) levels revealed that inoculation with ST induced a significant antibody response to antigens against ST, SE, and SG. Moreover, significant lymphoproliferative responses to the 3 Salmonella serovars were observed in the immunized group. We also investigated protection against virulent ST, SE, and SG strain challenge. Upon virulent SG challenge, the immunized group showed significantly reduced mortality compared to the non-immunized group. The reduced persistence of the virulent ST and SE challenge strains in the liver, spleen, and cecal tissues of the immunized group suggests that immunization with the attenuated ST strain may not only protect against ST infection but can also confer cross protection against SE and SG infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Silva EN, Duarte A. Salmonella Enteritidis in poultry: retrospective in Brazil. Braz J Poult Sci. 2002; 4:85–100.2. Forshell LP, Wierup M. Salmonella contamination: a significant challenge to the global marketing of animal food products. Rev Sci Tech. 2006; 25:541–554.3. Duguid JP, North RA. Eggs and Salmonella food-poisoning: an evaluation. J Med Microbiol. 1991; 34:65–72.4. Rodrigue DC, Tauxe RV, Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990; 105:21–27.5. Poppe C. Salmonella Infections in the Domestic Fowl. In : Wray C, Wray A, editors. Salmonella in domestic animals. Wallingford: CABI Publishing;2000. p. 107–132.6. Carvajal BG, Methner U, Pieper J, Berndt A. Effects of Salmonella enterica serovar Enteritidis on cellular recruitment and cytokine gene expression in caecum of vaccinated chickens. Vaccine. 2008; 26:5423–5433.
Article7. Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. The immunobiology of avian systemic salmonellosis. Vet Immunol Immunopathol. 2009; 128:53–59.
Article8. Velge P, Cloeckaert A, Barrow P. Emergence of Salmonella epidemics: the problems related to Salmonella enterica serotype Enteritidis and multiple antibiotic resistance in other major serotypes. Vet Res. 2005; 36:267–288.
Article9. McGruder ED, Ray PM, Tellez GI, Kogut MH, Corrier DE, DeLoach JR, Hargis BM. Salmonella enteritidis immune leukocyte-stimulated soluble factors: effects on increased resistance to Salmonella organ invasion in day-old Leghorn chicks. Poult Sci. 1993; 72:2264–2271.
Article10. Wigley P, Hulme S, Powers C, Beal R, Smith A, Barrow P. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet Res. 2005; 1:2.11. Girard MP, Steele D, Chaignat CL, Kieny MP. A review of vaccine research and development: human enteric infections. Vaccine. 2006; 24:2732–2750.
Article12. Sieve AN, Meeks KD, Bodhankar S, Lee S, Kolls JK, Simecka JW, Berg RE. A novel IL-17-dependent mechanism of cross protection: respiratory infection with mycoplasma protects against a secondary listeria infection. Eur J Immunol. 2009; 39:426–438.
Article13. Calenge F, Kaiser P, Vignal A, Beaumont C. Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: a review. Genet Sel Evol. 2010; 42:11.14. Gantois I, Ducatelle R, Timbermont L, Boyen F, Bohez L, Haesebrouck F, Pasmans F, van Immerseel F. Oral immunisation of laying hens with the live vaccine strains of TAD Salmonella vac E and TAD Salmonella vac T reduces internal egg contamination with Salmonella Enteritidis. Vaccine. 2006; 24:6250–6255.
Article15. Pathangey L, Kohler JJ, Isoda R, Brown TA. Effect of expression level on immune responses to recombinant oral Salmonella enterica serovar Typhimurium vaccines. Vaccine. 2009; 27:2707–2711.
Article16. Tan S, Gyles CL, Wilkie BN. Evaluation of an aroA mutant Salmonella typhimurium vaccine in chickens using modified semisolid Rappaport Vassiliadis medium to monitor faecal shedding. Vet Microbiol. 1997; 54:247–254.
Article17. Tellez GI, Kogut MH, Hargis BM. Immunoprophylaxis of Salmonella enteritidis infection by lymphokines in Leghorn chicks. Avian Dis. 1993; 37:1062–1070.
Article18. Chaudhari AA, Kim SW, Matsuda K, Lee JH. Safety evaluation and immunogenicity of arabinose-based conditional lethal Salmonella Gallinarum mutant unable to survive ex vivo as a vaccine candidate for protection against fowl typhoid. Avian Dis. 2011; 55:165–171.
Article19. Matsuda K, Chaudhari AA, Lee JH. Evaluation of safety and protection efficacy on cpxR and lon deleted mutant of Salmonella Gallinarum as a live vaccine candidate for fowl typhoid. Vaccine. 2011; 29:668–674.
Article20. Nandre RM, Matsuda K, Chaudhari AA, Kim B, Lee JH. A genetically engineered derivative of Salmonella Enteritidis as a novel live vaccine candidate for salmonellosis in chickens. Res Vet Sci. 2012; 93:596–603.
Article21. Matulova M, Havlickova H, Sisak F, Babak V, Rychlik I. SPI1 defective mutants of Salmonella enterica induce cross-protective immunity in chickens against challenge with serovars Typhimurium and Enteritidis. Vaccine. 2013; 31:3156–3162.
Article22. Wahid R, Simon R, Zafar SJ, Levine MM, Sztein MB. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin Vaccine Immunol. 2012; 19:825–834.
Article23. Mohler VL, Heithoff DM, Mahan MJ, Walker KH, Hornitzky MA, Shum LW, Makin KJ, House JK. Cross-protective immunity conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine in calves challenged with Salmonella serovar Newport. Vaccine. 2008; 26:1751–1758.
Article24. Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008; 26:1525–1529.
Article25. Beal RK, Wigley P, Powers C, Barrow PA, Smith AL. Cross-reactive cellular and humoral immune responses to Salmonella enterica serovars Typhimurium and Enteritidis are associated with protection to heterologous re-challenge. Vet Immunol Immunopathol. 2006; 114:84–93.
Article26. Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007; 25:6852–6862.
Article27. Chacana PA, Terzolo HR. Protection conferred by a live Salmonella Enteritidis vaccine against fowl typhoid in laying hens. Avian Dis. 2006; 50:280–283.
Article28. Roland K, Curtiss R III, Sizemore D. Construction and evaluation of a delta cya delta crp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 1999; 43:429–441.
Article29. Chaudhari AA, Lee JH. Evaluation of the adjuvant effect of Salmonella-based Escherichia coli heat-labile toxin B subunits on the efficacy of a live Salmonella-delivered avian pathogenic Escherichia coli vaccine. Avian Pathol. 2013; 42:365–372.
Article30. Chaudhari AA, Matsuda K, Lee JH. Construction of an attenuated Salmonella delivery system harboring genes encoding various virulence factors of avian pathogenic Escherichia coli and its potential as a candidate vaccine for chicken colibacillosis. Avian Dis. 2013; 57:88–96.
Article31. Dueger EL, House JK, Heithoff DM, Mahan MJ. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect Immun. 2001; 69:7950–7954.
Article32. Barrow PA, Lovell MA, Berchieri A. Immunisation of laying hens against Salmonella enteritidis with live attenuated vaccines. Vet Rec. 1990; 126:241–242.33. Nassar TJ, al-Nakhli HM, al-Ogaily ZH. Use of live and inactivated Salmonella enteritidis phage type 4 vaccines to immunise laying hens against experimental infection. Rev Sci Tech. 1994; 13:855–867.
Article34. Kim SW, Moon KH, Baik HS, Kang HY, Kim SK, Bahk JD, Hur J, Lee JH. Changes of physiological and biochemical properties of Salmonella enterica serovar Typhimurium by deletion of cpxR and lon genes using allelic exchange method. J Microbiol Methods. 2009; 79:314–320.
Article35. Lindberg AA, Segall T, Weintraub A, Stocker BA. Antibody response and protection against challenge in mice vaccinated intraperitoneally with a live aroA O4-O9 hybrid Salmonella dublin strain. Infect Immun. 1993; 61:1211–1221.
Article36. Alvarez J, Sota M, Vivanco AB, Perales I, Cisterna R, Rementeria A, Garaizar J. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J Clin Microbiol. 2004; 42:1734–1738.
Article37. Betancor L, Schelotto F, Fernandez M, Pereira M, Rial A, Chabalgoity JA. An attenuated Salmonella Enteritidis strain derivative of the main genotype circulating in Uruguay is an effective vaccine for chickens. Vet Microbiol. 2005; 107:81–89.
Article38. Porter RE Jr, Holt PS. Use of a pilocarpine-based lavage procedure to study secretory immunoglobulin concentration in the alimentary tract of White Leghorn chickens. Avian Dis. 1992; 36:529–536.
Article39. Gogal RM Jr, Ahmed SA, Larsen CT. Analysis of avian lymphocyte proliferation by a new, simple, nonradioactive assay (lympho-pro). Avian Dis. 1997; 41:714–725.
Article40. Centers for Disease Control and Prevention (CDC). Outbreak of Salmonella serotype Typhimurium associated with eating ground beef--Wisconsin. JAMA. 1996; 275:353–354.41. Roels TH, Frazak PA, Kazmierczak JJ, Mackenzie WR, Proctor ME, Kurzynski TA, Davis JP. Incomplete sanitation of a meat grinder and ingestion of raw ground beef: contributing factors to a large outbreak of Salmonella Typhimurium infection. Epidemiol Infect. 1997; 119:127–134.
Article42. Abd El Ghany M, Jansen A, Clare S, Hall L, Pickard D, Kingsley RA, Dougan G. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun. 2007; 75:1835–1842.
Article43. Kotton CN, Hohmann EL. Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect Immun. 2004; 72:5535–5547.
Article44. Park JH, Sung HW, Yoon BI, Kwon HM. Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-gamma. J Vet Sci. 2009; 10:131–139.
Article45. Rana N, Kulshreshtha RC. Cell-mediated and humoral immune responses to a virulent plasmid-cured mutant strain of Salmonella enterica serotype gallinarum in broiler chickens. Vet Microbiol. 2006; 115:156–162.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidrug-resistant Salmonella typhimurium and Salmonella enteritidis Identified by Multiplex PCR in Korea
- Establishment of a live vaccine strain against fowl typhoid and paratyphoid
- In vitro infection of murine macrophages with salmonella typhimurium and listeria monocytogenes
- Electron microscopic study on the response of the intestinal mucosa and macrophage to invasion of salmonella typhimurium
- Competitive exclusion against Salmonella gallinarum of Salmonella enteritidis infected chickens