Immune Netw.
2011 Apr;11(2):123-133. 10.4110/in.2011.11.2.123.
Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-kappaB, and MAPK Pathways
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Daejeon 301-747, Korea. hayoungj@cnu.ac.kr
- 2Infection Signaling Network Research Center, College of Medicine, Chungnam National University, Daejeon 301-747, Korea.
- KMID: 2150700
- DOI: http://doi.org/10.4110/in.2011.11.2.123
Abstract
- BACKGROUND
Mycobacterium tuberculosis (Mtb) heparin binding hemagglutinin (HBHA) is an Ag known to evoke effective host immune responses during tuberculosis infection. However, the molecular basis of the host immune response to HBHA has not been fully characterized. In this study, we examined the molecular mechanisms by which HBHA can induce the expression of proinflammatory cytokines in macrophages.
METHODS
HBHA-induced mRNA and protein levels of proinflammatory cytokines were determined in bone marrow-derived macrophages (BMDMs) using RT-PCR and ELISA analysis. The roles of intracellular signaling pathways for NF-kappaB, PI3-K/Akt, and MAPKs were investigated in macrophage proinflammatory responses after stimulation with HBHA.
RESULTS
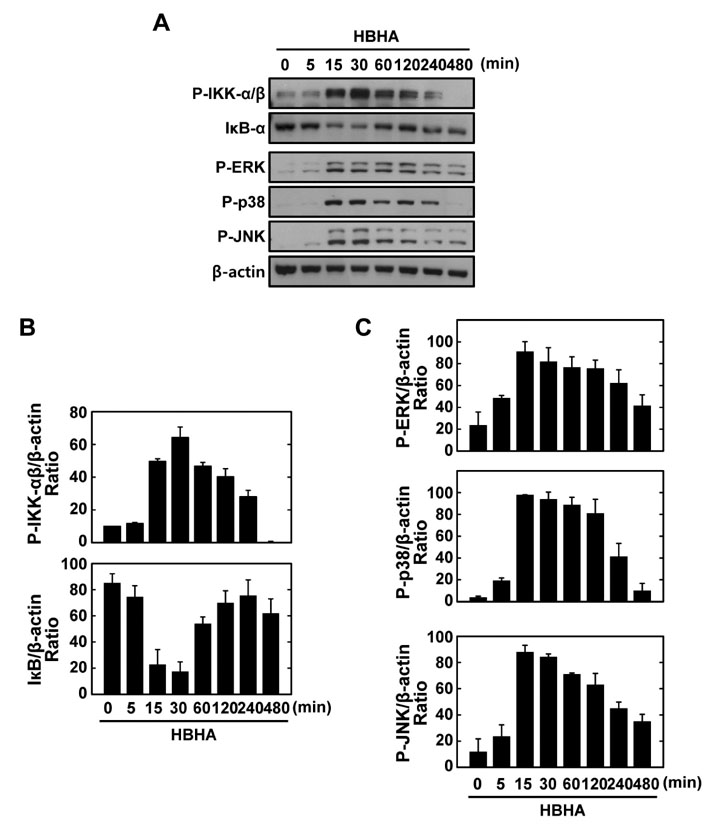
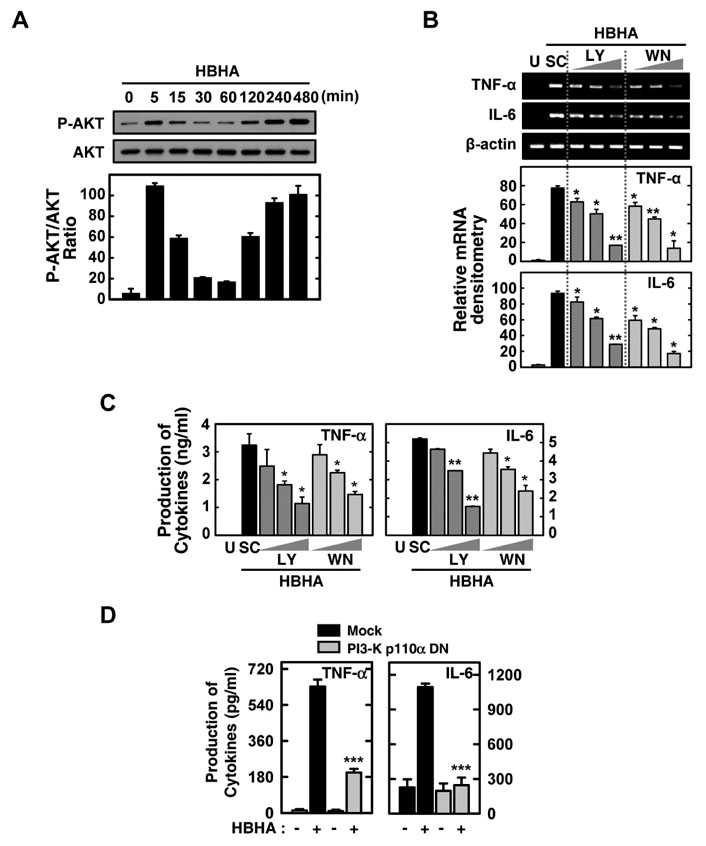
HBHA robustly activated the expression of mRNA and protein of both TNF-alpha and IL-6, and induced phosphorylation of NF-kappaB, Akt, and MAPKs in BMDMs. Both TNF-alpha and IL-6 production by HBHA was regulated by the NF-kappaB, PI3-K, and MAPK pathways. Furthermore, PI3-K activity was required for the HBHA-induced activation of ERK1/2 and p38 MAPK, but not JNK, pathways.
CONCLUSION
These data suggest that mycobacterial HBHA significantly induces proinflammatory responses through crosstalk between the PI3-K and MAPK pathways in macrophages.
Keyword
MeSH Terms
-
Cytokines
Enzyme-Linked Immunosorbent Assay
Hemagglutinins
Heparin
Interleukin-6
Lectins
Macrophages
Mycobacterium tuberculosis
NF-kappa B
p38 Mitogen-Activated Protein Kinases
Phosphorylation
RNA, Messenger
Tuberculosis
Tumor Necrosis Factor-alpha
Cytokines
Hemagglutinins
Heparin
Interleukin-6
Lectins
NF-kappa B
RNA, Messenger
Tumor Necrosis Factor-alpha
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria:branching out from Toll-like receptors. Cell Microbiol. 2007. 9:1087–1098.
Article2. Menozzi FD, Bischoff R, Fort E, Brennan MJ, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci U S A. 1998. 95:12625–12630.
Article3. Pethe K, Bifani P, Drobecq H, Sergheraert C, Debrie AS, Locht C, Menozzi FD. Mycobacterial heparin-binding hemagglutinin and laminin-binding protein share antigenic methyllysines that confer resistance to proteolysis. Proc Natl Acad Sci U S A. 2002. 99:10759–10764.
Article4. Menozzi FD, Rouse JH, Alavi M, Laude-Sharp M, Muller J, Bischoff R, Brennan MJ, Locht C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996. 184:993–1001.
Article5. Parra M, Pickett T, Delogu G, Dheenadhayalan V, Debrie AS, Locht C, Brennan MJ. The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis. Infect Immun. 2004. 72:6799–6805.
Article6. Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis:myths and truth. Microbes Infect. 2008. 10:995–1004.7. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999. 285:732–736.
Article8. Toossi Z. Cytokine circuits in tuberculosis. Infect Agents Dis. 1996. 5:98–107.9. Bermudez LE, Young LS. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988. 140:3006–3013.10. Lee JS, Song CH, Lim JH, Kim HJ, Park JK, Paik TH, Kim CH, Kong SJ, Shon MH, Jung SS, Jo EK. The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 2003. 132:443–449.
Article11. Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, Song CH, Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006. 74:2686–2696.
Article12. Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008. 10:741–754.
Article13. Alemán M, Schierloh P, de la Barrera SS, Musella RM, Saab MA, Baldini M, Abbate E, Sasiain MC. Mycobacterium tuberculosis triggers apoptosis in peripheral neutrophils involving toll-like receptor 2 and p38 mitogen protein kinase in tuberculosis patients. Infect Immun. 2004. 72:5150–5158.
Article14. Delogu G, Bua A, Pusceddu C, Parra M, Fadda G, Brennan MJ, Zanetti S. Expression and purification of recombinant methylated HBHA in Mycobacterium smegmatis. FEMS Microbiol Lett. 2004. 239:33–39.
Article15. Shin AR, Lee KS, Lee JS, Kim SY, Song CH, Jung SB, Yang CS, Jo EK, Park JK, Paik TH, Kim HJ. Mycobacterium tuberculosis HBHA protein reacts strongly with the serum immunoglobulin M of tuberculosis patients. Clin Vaccine Immunol. 2006. 13:869–875.
Article16. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994. 12:141–179.17. Pathak SK, Bhattacharyya A, Pathak S, Basak C, Mandal D, Kundu M, Basu J. Toll-like receptor 2 and mitogen- and stress-activated kinase 1 are effectors of Mycobacterium avium-induced cyclooxygenase-2 expression in macrophages. J Biol Chem. 2004. 279:55127–55136.
Article18. Yadav M, Roach SK, Schorey JS. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol. 2004. 172:5588–5597.
Article19. Lee HM, Shin DM, Kim KK, Lee JS, Paik TH, Jo EK. Roles of reactive oxygen species in CXCL8 and CCL2 expression in response to the 30-kDa antigen of Mycobacterium tuberculosis. J Clin Immunol. 2009. 29:46–56.
Article20. Jung SB, Song CH, Yang CS, Kim SY, Lee KS, Shin AR, Lee JS, Nam HS, Kim HJ, Park JK, Paik TH, Jo EK. Role of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in the secretion of tumor necrosis factor-alpha and interleukin-10 by the PPD antigen of Mycobacterium tuberculosis. J Clin Immunol. 2005. 25:482–490.
Article21. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection:the MAP kinases lead the way. Cell Microbiol. 2003. 5:133–142.
Article22. Darieva Z, Lasunskaia EB, Campos MN, Kipnis TL, Da Silva WD. Activation of phosphatidylinositol 3-kinase and c-Jun-N-terminal kinase cascades enhances NF-kappaB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding. J Leukoc Biol. 2004. 75:689–697.
Article23. Sendide K, Reiner NE, Lee JS, Bourgoin S, Talal A, Hmama Z. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria:regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005. 174:4210–4219.
Article24. Yang CS, Lee JS, Jung SB, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Differential regulation of interleukin-12 and tumour necrosis factor-alpha by phosphatidylinositol 3-kinase and ERK 1/2 pathways during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2006. 143:150–160.
Article25. Weir RE, Black GF, Dockrell HM, Floyd S, Fine PE, Chaguluka SD, Stenson S, King E, Nazareth B, Warndorff DK, Ngwira B, Crampin AC, Mwaungulu L, Sichali L, Jarman E, Donovan L, Blackwell JM. Mycobacterial purified protein derivatives stimulate innate immunity:Malawians show enhanced tumor necrosis factor alpha, interleukin-1beta (IL-1beta), and IL-10 responses compared to those of adolescents in the United Kingdom. Infect Immun. 2004. 72:1807–1811.
Article26. Wallis RS, Amir-Tahmasseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins:the monocyte western blot. Proc Natl Acad Sci U S A. 1990. 87:3348–3352.
Article27. Temmerman S, Pethe K, Parra M, Alonso S, Rouanet C, Pickett T, Drowart A, Debrie AS, Delogu G, Menozzi FD, Sergheraert C, Brennan MJ, Mascart F, Locht C. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat Med. 2004. 10:935–941.
Article28. Hougardy JM, Schepers K, Place S, Drowart A, Lechevin V, Verscheure V, Debrie AS, Doherty TM, Van Vooren JP, Locht C, Mascart F. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007. 2:e926.29. Locht C, Hougardy JM, Rouanet C, Place S, Mascart F. Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis (Edinb). 2006. 86:303–309.
Article30. Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001. 193:239–246.
Article31. Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008. 8:837–848.32. Wang T, Lafuse WP, Zwilling BS. NFkappaB and Sp1 elements are necessary for maximal transcription of toll-like receptor 2 induced by Mycobacterium avium. J Immunol. 2001. 167:6924–6932.
Article33. Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005. 280:20961–20967.
Article34. Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001. 291:1544–1547.
Article35. Maiti D, Bhattacharyya A, Basu J. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001. 276:329–333.
Article36. Vanhaesebroeck B, Jones GE, Allen WE, Zicha D, Hooshmand-Rad R, Sawyer C, Wells C, Waterfield MD, Ridley AJ. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat Cell Biol. 1999. 1:69–71.
Article37. Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001. 276:35482–35493.
Article38. Lee JS, Son JW, Jung SB, Kwon YM, Yang CS, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Ex vivo responses for interferon-gamma and proinflammatory cytokine secretion to low-molecular-weight antigen MTB12 of Mycobacterium tuberculosis during human tuberculosis. Scand J Immunol. 2006. 64:145–154.
Article39. Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003. 23:194–201.40. Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006. 177:6317–6324.
Article41. Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002. 277:32124–32132.
Article42. Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007. 104:4077–4082.
Article43. Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ. Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996. 271:12133–12136.
Article44. Jung ID, Jeong SK, Lee CM, Noh KT, Heo DR, Shin YK, Yun CH, Koh WJ, Akira S, Whang J, Kim HJ, Park WS, Shin SJ, Park YM. Enhanced Efficacy of Therapeutic Cancer Vaccines Produced by Co-Treatment with Mycobacterium tuberculosis Heparin-Binding Hemagglutinin, a Novel TLR4 Agonist. Cancer Res. 2011. 71:2858–2870.
Article45. Place S, Verscheure V, de San N, Hougardy JM, Schepers K, Dirix V, Dediste A, Michel O, Drowart A, Allard SD, Doherty TM, Lecher S, Locht C, Mascart F. Heparin-binding, hemagglutinin-specific IFN-gamma synthesis at the site of infection during active tuberculosis in humans. Am J Respir Crit Care Med. 2010. 182:848–854.
Article46. Zanetti S, Bua A, Delogu G, Pusceddu C, Mura M, Saba F, Pirina P, Garzelli C, Vertuccio C, Sechi LA, Fadda G. Patients with pulmonary tuberculosis develop a strong humoral response against methylated heparin-binding hemagglutinin. Clin Diagn Lab Immunol. 2005. 12:1135–1138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection
- NF-kappaB Activation in T Helper 17 Cell Differentiation
- Role of PI3K/Akt Pathway in the Activation of IkappaB/NF-kappaB Pathway in Lung Epithelial Cells
- Anti-inflammatory activity of compounds isolated from Astragalus sinicus L. in cytokine-induced keratinocytes and skin