Immune Netw.
2011 Feb;11(1):50-58. 10.4110/in.2011.11.1.50.
Expression of Epstein-Barr Virus Gene and Clonality of Infiltrated T Lymphocytes in Epstein-Barr Virus-associated Gastric Carcinoma
- Affiliations
-
- 1Department of Microbiology, Brain Korea Project 21 of Medical Sciences, Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul 120-752, Korea. jhpark5277@yumc.yonsei.ac.kr
- 2Department of Pathology, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 3Department of Surgery, Yonsei University College of Medicine, Seoul 120-752, Korea.
- KMID: 2150692
- DOI: http://doi.org/10.4110/in.2011.11.1.50
Abstract
- BACKGROUND
Epstein-Barr virus associated gastric lymphoepithelioma-like carcinoma (LELC) is characterized by the intensive infiltration of lymphoid cells, the presence of EBV, and the better prognosis over typical adenocarcinoma. Thus, it was assumable that viral latent proteins may be responsible for the recruitment of a certain T cell repertoire to EBV-associated gastric carcinoma.
METHODS
To examine above possibility, EBV gene expression in gastric carcinoma tissues and usage of TCR among the tumor infiltrating lymphocytes were analyzed.
RESULTS
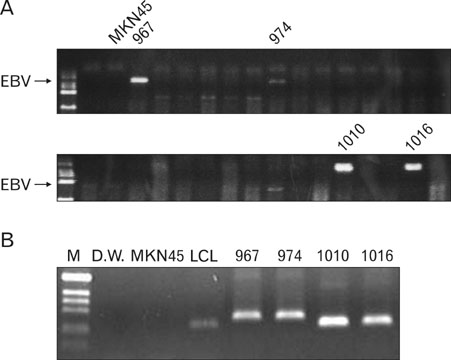
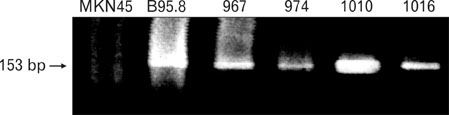
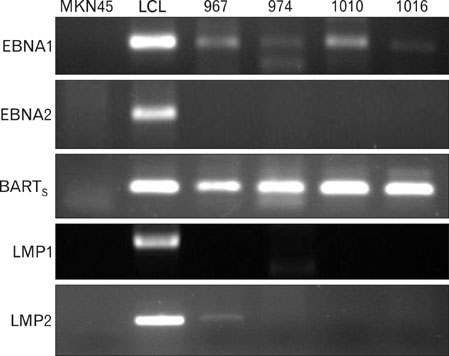
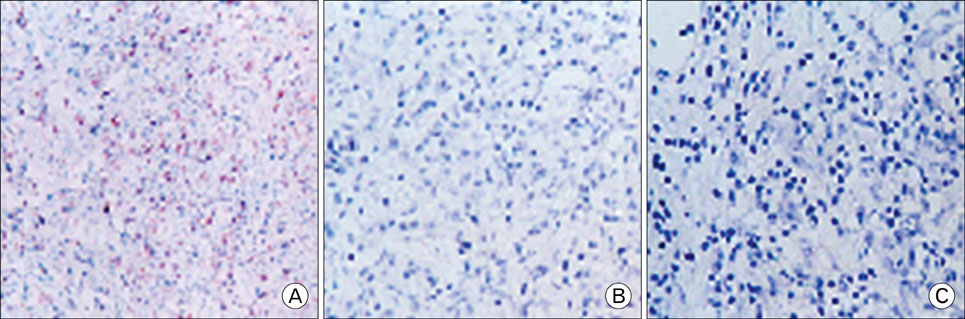
EBV specific DNA and EBERs RNA were detected in 4 out of 30 patients. RT-PCR analysis revealed that all 4 of EBV-positive tumor tissues expressed EBNA1 mRNA and BARTs and LMP2a was detected only one sample out of 4. However, the EBNA2 and LMP-1 transcripts were not detected in these tissues. CD8+ T cells were the predominant population of infiltrating lymphocytes in the EBV-positive gastric carcinoma. According to spectra type analysis of infiltrating T cells, 10 predominant bands were detected by TCR Vbeta CDR3 specific RT-PCR from 4 EBV-positive tumor tissues. Sequence analysis of these bands revealed oligoclonal expansion of T cells.
CONCLUSION
These findings suggest that clonally expanded T cells in vivo might be a population of cytotoxic T cells reactive to EBV-associated gastric carcinoma.
MeSH Terms
Figure
Cited by 1 articles
-
Current Trends in Studies of Epstein-Barr Virus (EBV) Associated Gastric Carcinoma
Minjung Lee, Eunhyun Ryu, Gi-Ho Sung, Yu Su Shin, Jong Gwang Kim, Byung Woog Kang, Hyosun Cho, Hyojeung Kang
J Bacteriol Virol. 2015;45(3):262-271. doi: 10.4167/jbv.2015.45.3.262.
Reference
-
1. Rickinson AB, Kieff E. Knipe DM, Howley PM. Epstein-Barr virus. Fields virology. 2007. 5th ed. Philadelphia: Lippincott Williams & Wilkins;2655–2700.2. Kieff E, Rickinson AB. Fields BN, Knipe DM, Howley PM. Epstein-Barr Virus and its replication. Fields Virology. 2007. 5th ed. Philadelphia: Lippincott-Williams & Wilkins Publishers;2603–2654.3. Sample J, Brooks L, Sample C, Young L, Rowe M, Gregory C, Rickinson A, Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci U S A. 1991. 88:6343–6347.
Article4. Young LS, Murray PG. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene. 2003. 22:5108–5121.
Article5. Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996. 183:725–729.
Article6. Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997. 15:405–431.
Article7. Kienzle N, Sculley TB, Poulsen L, Buck M, Cross S, Raab-Traub N, Khanna R. Identification of a cytotoxic T-lymphocyte response to the novel BARF0 protein of Epstein-Barr virus: a critical role for antigen expression. J Virol. 1998. 72:6614–6620.
Article8. Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla MG, Frappier L, Rickinson A. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997. 7:791–802.
Article9. Yin Y, Manoury B, Fåhraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003. 301:1371–1374.
Article10. Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008. 99:195–201.
Article11. Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990. 3:377–380.12. Herath CH, Chetty R. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Arch Pathol Lab Med. 2008. 132:706–709.
Article13. Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994. 73:2239–2249.
Article14. Corvalan A, Ding S, Koriyama C, Carrascal E, Carrasquilla G, Backhouse C, Urzua L, Argandoña J, Palma M, Eizuru Y, Akiba S. Association of a distinctive strain of Epstein-Barr virus with gastric cancer. Int J Cancer. 2006. 118:1736–1742.
Article15. Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y, Ueda H, Ogino T, Nakanishi I. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer. 1990. 66:945–952.
Article16. van Beek J, zur Hausen A, Snel SN, Berkhof J, Kranenbarg EK, van de Velde CJ, van den Brule AJ, Middeldorp JM, Meijer CJ, Bloemena E. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. Am J Surg Pathol. 2006. 30:59–65.
Article17. Sample J, Young L, Martin B, Chatman T, Kieff E, Rickinson A, Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990. 64:4084–4092.
Article18. Sugiura M, Imai S, Tokunaga M, Koizumi S, Uchizawa M, Okamoto K, Osato T. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer. 1996. 74:625–631.
Article19. Choi IH, Chwae YJ, Shim WS, Kim DS, Kwon DH, Kim JD, Kim SJ. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997. 159:481–486.20. Hall BL, Finn OJ. PCR-based analysis of the T-cell receptor V beta multigene family: experimental parameters affecting its validity. Biotechniques. 1992. 13:248–257.21. Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992. 140:769–774.22. Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000. 53:255–261.
Article23. Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996. 27:20–27.
Article24. van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ, Bloemena E. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004. 22:664–670.
Article25. Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A. 1994. 91:9131–9135.
Article26. Shin WS, Kang MW, Kang JH, Choi MK, Ahn BM, Kim JK, Sun HS, Min KW. Epstein-Barr virus-associated gastric adenocarcinomas among Koreans. Am J Clin Pathol. 1996. 105:174–181.
Article27. Sixbey JW, Shirley P, Chesney PJ, Buntin DM, Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989. 2:761–765.
Article28. Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol Int. 2010. 60:337–350.
Article29. Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J Exp Med. 1997. 185:1605–1617.
Article30. Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen PM, Klein G, Kurilla MG, Masucci MG. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995. 375:685–688.
Article31. Münz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O'Donnell M, Steinman RM. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000. 191:1649–1660.
Article32. Roskrow MA, Suzuki N, Gan Y, Sixbey JW, Ng CY, Kimbrough S, Hudson M, Brenner MK, Heslop HE, Rooney CM. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease. Blood. 1998. 91:2925–2934.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on the Epstein-Barr virus transformed human B-lymphocytes1. flowcytometry analysis of Epstein-Barr virus transformed human B-lymphocytes
- Studies on the Epstein-Barr virus transformed human B-lymphocytes2. production of LT-like factor by Epstein-Barr virus transformed human B-lymphocytes
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Epstein-Barr Virus in Human Malignancy: A Special Reference to Epstein-Barr Virus associated Gastric Carcinoma
- A Case of Epstein-Barr Virus-Associated Hemophagocytic Syndrome Demonstrated by In Situ Hybridization