Immune Netw.
2010 Dec;10(6):230-238. 10.4110/in.2010.10.6.230.
Activation of Macrophages by Exopolysaccharide Produced by MK1 Bacterial Strain Isolated from Neungee Mushroom, Sarcodon aspratus
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea.
- 2Department of Microbiology, College of Natural Sciences, Chungbuk National University, Cheongju 361-763, Korea. ynlee@chungbuk.ac.kr
- KMID: 2150679
- DOI: http://doi.org/10.4110/in.2010.10.6.230
Abstract
- BACKGROUND
The MK1 strain, a novel bacterial isolate from soft-rotten tissue of the Neungee mushroom, produces copious amounts of exopolysaccharide (EPS) in a dextrose minimal medium. This study examined the molecular characteristics and immunomodulatory activity of MK1 EPS.
METHODS
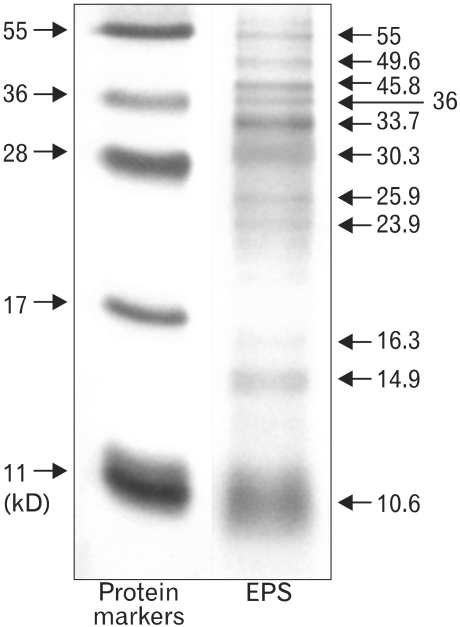
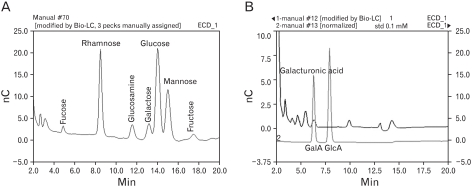
The EPS in the culture supernatant was purified by cold ethanol precipitation, and characterized by SDS-PAGE/silver staining and Bio-HPLC. The immunomodulatory activities of the EPS were examined using the mouse monocytic cell line, RAW 264.7 cells.
RESULTS
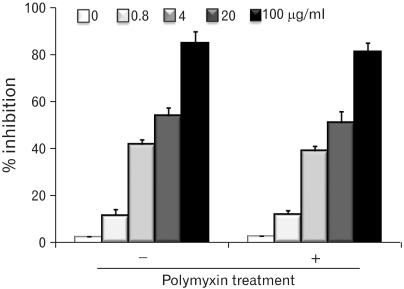
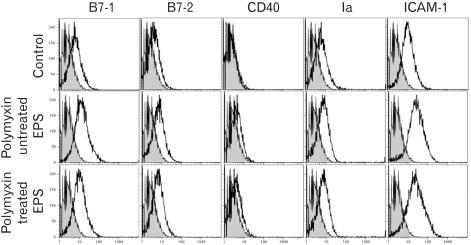
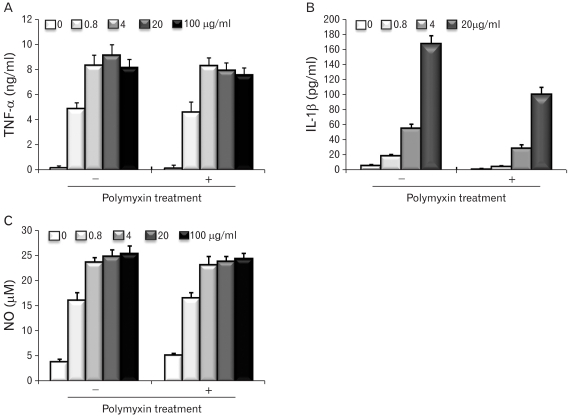
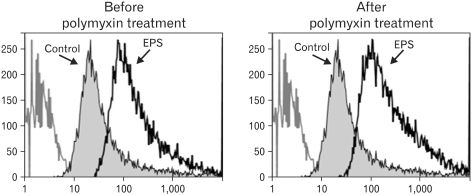
The molecular weights of the purified EPS were rather heterogeneous, ranging from 10.6 to 55 kDa. The EPS was composed of glucose, rhamnose, mannose, galactose, and glucosamine at an approximate molar ratio of 1.00:0.8:0.71:0.29:0.21. EPS activated the RAW cells to produce cytokines, such as TNF-alpha and IL-1beta, and nitric oxide (NO). EPS also induced the expression of co-stimulatory molecules, such as B7-1, B7-2 and ICAM-1, and increased the phagocytic activity. The macrophage-activating activity of EPS was not due to endotoxin contamination because the treatment of EPS with polymyin B did not reduce the macrophage-activating activity.
CONCLUSION
The EPS produced from the MK1 strain exerts macrophage-activating activity.
MeSH Terms
-
Agaricales
Animals
Cell Line
Cold Temperature
Cytokines
Ethanol
Galactose
Glucosamine
Glucose
Intercellular Adhesion Molecule-1
Macrophages
Mannose
Mice
Molar
Molecular Weight
Nitric Oxide
Rhamnose
Sprains and Strains
Tumor Necrosis Factor-alpha
Cytokines
Ethanol
Galactose
Glucosamine
Glucose
Intercellular Adhesion Molecule-1
Mannose
Nitric Oxide
Rhamnose
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Welman AD, Maddox IS. Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 2003; 21:269–274. PMID: 12788547.
Article2. Sutherland IW. Bacterial exopolysaccharides. Adv Microb Physiol. 1972; 8:143–213. PMID: 4581483.
Article3. Wang H, Jiang X, Mu H, Liang X, Guan H. Structure and protective effect of exopolysaccharide from P. Agglomerans strain KFS-9 against UV radiation. Microbiol Res. 2007; 162:124–129. PMID: 16580187.
Article4. Sutherland I. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology. 2001; 147:3–9. PMID: 11160795.
Article5. Kumar CG, Joo HS, Choi JW, Koo YM, Chang CS. Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb Technol. 2004; 34:673–681.
Article6. Iyer A, Mody K, Jha B. Rheological properties of an exopolysaccharide produced by a marine Enterobacter cloaceae. Natl Acad Sci Lett. 2005; 28:119–123.7. Lin CC, Casida LE. GELRITE as a gelling agent in media for the growth of thermophilic microorganisms. Appl Environ Microbiol. 1984; 47:427–429. PMID: 16346480.
Article8. Kalogiannis S, Iakovidou G, Liakopoulou-Kyriakides M, Kyriakidis DA, Skaracis GN. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem. 2003; 39:249–256.
Article9. Kong J, Lee H, Hong J, Kang Y, Kim J, Chang M, Bae S. Utilization of a cell-bound polysaccharide produced by the marine bacterium Zooglea sp: new biomaterial for metal adsorption and enzyme immobilization. J Mar Biotechnol. 1998; 6:99–103.10. Okutani K. Antitumor and immunostimulant activities of polysaccharides produced by a marine bacterium of the genus Vibrio. Bull Jap Soc Sci Fish. 1984; 50:1035–1037.11. Okutani K. Antiviral activities of sulfated derivatives of a fucosamine-containing polysaccharide of marine bacterial origin. Nippon Suisan Gakkaishi. 1992; 58:927–930.
Article12. Abbad Andaloussi S, Talbaoui H, Marczak R, Bonaly R. Isolation and characterization of exocellular polysaccharides produced by Bifidobacterium longum. Appl Microbiol Biotechnol. 1995; 43:995–1000. PMID: 8590666.
Article13. Roberts CM, Felt WF, Osman SF, Wijey C, O'Connor JV, Hoover DG. Exopolysaccharide production by Bifidobacterium longum BB-79. J Appl Bacteriol. 1995; 78:463–468.14. Sreekumar O, Hosono A. The antimutagenic properties of a polysaccharide produced by Bifidobacterium longum and its cultured milk against some heterocyclic amines. Can J Microbiol. 1998; 44:1029–1036. PMID: 10029998.
Article15. Arena A, Maugeri TL, Pavone B, Iannello D, Gugliandolo C, Bisignano G. Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int Immunopharmacol. 2006; 6:8–13. PMID: 16332508.
Article16. Kitazawa H, Yamaguchi T, Itoh T. B-cell mitogenic activity of slime products produced from slime-forming, encapsulated Lactococcus lactis ssp. cremoris. J Dairy Sci. 1992; 75:2946–2951. PMID: 1460126.
Article17. Kitazawa H, Yamaguchi T, Miura M, Saito T, Itoh T. B-cell mitogen produced by slime-forming, encapsulated Lactococcus lactis ssp. cremoris isolated from ropy sour milk, viili. J Dairy Sci. 1993; 76:1514–1519. PMID: 8326024.
Article18. Kitazawa H, Yamaguchi T, Fujimoto Y, Itoh T. Comparative activity of B-cell mitogen, a phosphopolysaccharide, produced by L. lactis ssp. cremoris on various lymphocytes. Anim Sci Technol. 1993; 64:605–607.19. Kitazawa H, Yamaguchi T, Fujimoto Y, Itoh T. An analysis of mitogenic response of phosphopolysaccharide, a B-cell mitogen produced by Lactococcus lactis ssp. cremoris to spleen cells. Anim Sci Technol. 1993; 64:807–812.20. Kitazawa H, Itoh T, Yamaguchi T. Induction of macrophage cytotoxicity by slime products produced by encapsulated Lactococcus lactis ssp. cremoris. Anim Sci Technol. 1991; 62:861–866.21. Kitazawa H, Itoh T, Tomioka Y, Mizugaki M, Yamaguchi T. Induction of IFNγ and IL-1α production in macrophages stimulated with phosphopolysaccharide produced by Lactococcus lactis ssp. cremoris. Int J Food Microbiol. 1996; 31:99–106. PMID: 8880300.22. Lee YN, Koo CD. Identification of bacteria isolated from diseased Neungee mushroom, Sarcodon aspratus. J Basic Microbiol. 2007; 47:31–39. PMID: 17304616.23. Lee YN, Kim EJ. Characterization of a novel bacterium isolated from diseased Nuengee mushroom, Sarcodon aspratus. 2010. In : 11th Int Sym on the Genetics of Industrial Microorganisms; Melbourne, Australia. –Abs. p29.24. Ryu JE, Lee YN. Optimizing culture condition of MK1 strain isolated from soft-rotten tissue of Neungee mushroom and its exopolysaccharide. Korean J Microbiol. 2009; 45:324–331.25. McKellar RC, Geest JV, Cui W. Influence of culture and environmental conditions on the composition of exopolysaccharide produced by Agrobacterium radiobacter. Food Hydrocolloides. 2003; 17:429–437.
Article26. Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colormetric method for the determination of sugars. Nature. 1951; 168:167. PMID: 14875032.27. Masuko T, Minami A, Iwasaki N, Majima T, Nishimura SI, Lee YC. Carbohydrate analysis by a phenol-sulfuric and method in microplate format. Anal Biochem. 2005; 339:69–72. PMID: 15766712.28. Ramus J. Alcian blue: A quantitative aqueous assay for algal and sulfated polysaccharides. J Phycol. 1977; 13:345–348.29. Laemmli EK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–685. PMID: 5432063.
Article30. Kim JB, Ahn JH. The modification of the silver stain method in sodium dodecyl sulfate-polyamide gels for detecting lipopolysaccharides. J Korean Soc Microbiol. 1993; 28:193–198.31. Hedrick JL, Smith AJ. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968; 126:155–164. PMID: 5671059.
Article32. Lee JK, Lee MK, Yun Y-P, Kim Y, Kim JS, Kim YS, Kim K, Han SS, Lee CK. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int Immunopharmacol. 2001; 1:1275–1284. PMID: 11460308.
Article33. Gerelchuluun T, Lee YH, Lee YR, Im SA, Song S, Park JS, Han K, Kim K, Lee CK. Dendritic cells process antigens encapsulated in a biodegradable polymer, poly (D,L-lactide-co-glycolide), via an alternate class I MHC processing pathway. Arch Pharm Res. 2007; 30:1440–1446. PMID: 18087813.34. Lorsbach RB, Murphy WJ, Lowenstein CJ, Snyder SH, Russell SW. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993; 268:1908–1913. PMID: 7678412.
Article35. Sherwin C, Fern R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-alpha, IL-1beta, and calcium. J Immunol. 2005; 175:155–161. PMID: 15972642.36. Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002; 22:3052–3060. PMID: 11943808.37. Im SA, Oh ST, Song S, Kim MR, Kim DS, Woo SS, Jo TH, Park YI, Lee CK. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int Immunopharmacol. 2005; 5:271–279. PMID: 15652758.
Article38. Yamada H, Kiyohara H, Cyong JC, Kojima Y, Kumazawa Y, Otsuka Y. Studies on polysaccharides from Angelica acutiloba. Part 1. Fractionation and biological properties of polysaccharides. Planta Med. 1984; 50:163–167.39. Pang ZJ, Zhou M, Chen Y, Wan J. A protein-bound polysaccharide synergistic with lipopolysaccharide induces nitric oxide release and antioxidant enzyme activities in mouse peritoneal macrophages. Am J Chin Med. 1998; 26:133–141. PMID: 9799965.
Article40. Okamura K, Suzuki M, Yajima A, Chihara T, Fujiwara A, Fukuda T, Goto S, Ichinohe K, Jimi S, Kasamatsu T, Kawai N, Mizuguchi K, Mori S, Nakano H, Noda K, Sekiba K, Suzuki K, Suzuki T, Takahashi K, Takeuchi K, Takeuchi S, Ogawa N. Clinical evaluation of schizophyllan combined with irradiation in patients with cervical cancer. A randomized controlled study. Cancer. 1986; 58:865–872. PMID: 2941141.
Article41. Sasaki T, Takasuka N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr Res. 1976; 47:99–104. PMID: 178444.
Article42. Kitazawa H, Harata T, Uemura J, Saito T, Kaneko T, Itoh T. Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii subsp. bulgaricus. Int J Food Microbiol. 1998; 40:169–175. PMID: 9620124.43. Chen NY, Hsu TH, Lin FY, Lai HH, Wu JY. Effects on cytokine-stimulating activities of EPS from Tremella mesenterica with various carbon sources. Food Chem. 2006; 99:92–97.
Article44. Chabot S, Yu HL, Leseleuc LD, Cloitier D, Van Calsteren MR, Lessard M, Roy D, Lacroix M, Oth D. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M stimulate TNF, IL-6 and IL-12 in human and mouse cultured immunocompetent cells and IFN-γ in mouse splenocytes. Lait. 2001; 81:683–697.45. Cescutti P, Kallioinen A, Impallomeni G, Toffanin R, Pollesello P, Leisola M, Eerikainen T. Structure of exopolysaccharide produced by Enterobacter amnigenus. Carbohydr Res. 2005; 340:439–447. PMID: 15680599.46. Castro R, Piazzon MC, Zarra I, Leiro J, Noya M, Lamas J. Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture. 2006; 254:9–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Chitosan Acetate on Bacteria Occurring on Neungee Mushrooms, Sarcodon aspratus
- Effects of cytokines in the activation of peritoneal macrophages from mice infected with Toxoplasma gondii
- Non-specific activation of mouse peritoneal macrophages by a freshwater ciliate, Tetrahymena pyriformis
- Occurrence of Internal Stipe Necrosis of Cultivated Mushrooms (Agaricus bisporus) Caused by Ewingella americana in Korea
- Culture of tissue-cyst forming strain of Toxoplasma gondii and the effect of cyclic AMP and pyrimidine salvage inhibitors