Immune Netw.
2010 Apr;10(2):55-63. 10.4110/in.2010.10.2.55.
Immunostimulatory Effects of Cordyceps militaris on Macrophages through the Enhanced Production of Cytokines via the Activation of NF-kappaB
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, Seoul 139-742, Korea. kimkj@syu.ac.kr
- 2College of Pharmacy, Chungbuk University, Cheongju 361-763, Korea.
- 3Department of Biology, Seoul Women's University, Seoul 139-774, Korea.
- KMID: 2150660
- DOI: http://doi.org/10.4110/in.2010.10.2.55
Abstract
-
BACKGROUND: Cordyceps militaris has been used in traditional medicine to treat numerous diseases and has been reported to possess both antitumor and immunomodulatory activities in vitro and in vivo. However, the pharmacological and biochemical mechanisms of Cordyceps militaris extract (CME) on macrophages have not been clearly elucidated. In the present study, we examined how CME induces the production of proinflammatory cytokines, transcription factor, and the expression of co-stimulatory molecules.
METHODS
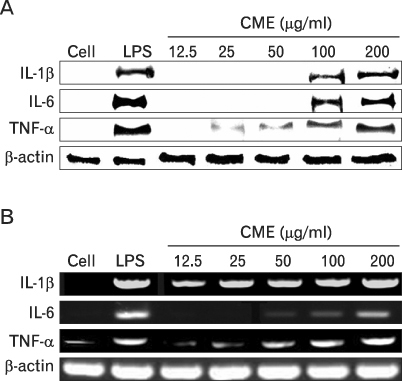
We confirmed the mRNA and protein levels of proinflammatory cytokines through RT-PCR and western blot analysis, followed by a FACS analysis for surface molecules.
RESULTS
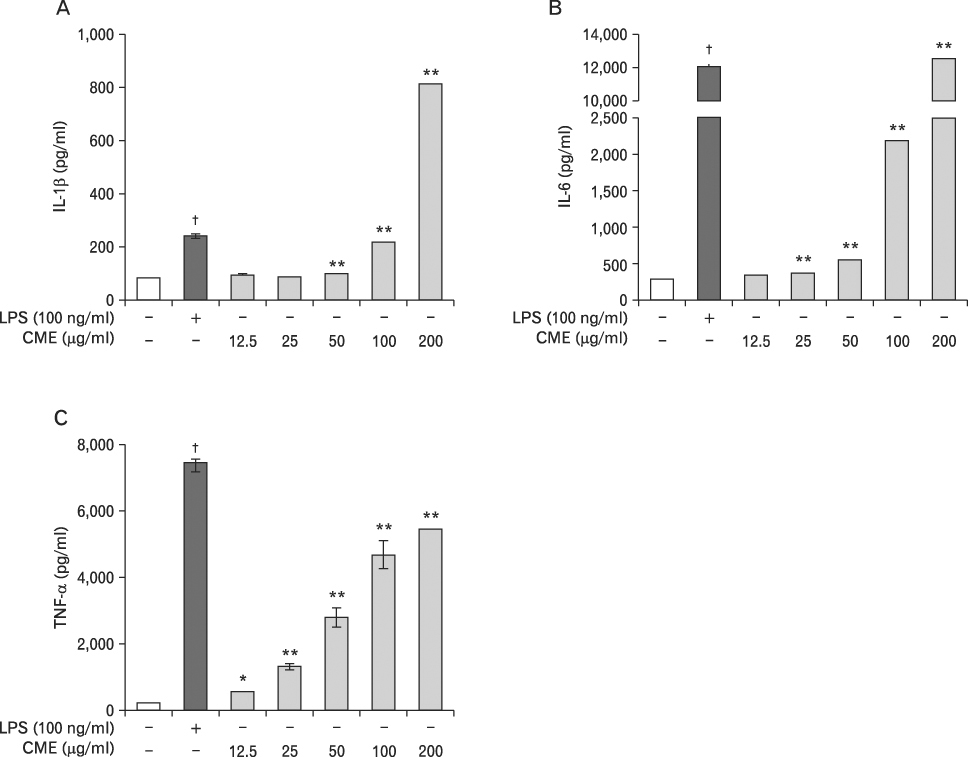
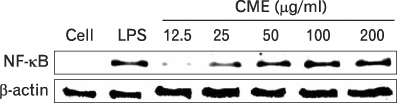
CME dose dependently increased the production of NO and proinflammatory cytokines such as IL-1beta, IL-6, TNF-alpha, and PGE(2), and it induced the protein levels of iNOS, COX-2, and proinflammatory cytokines in a concentration-dependent manner, as determined by western blot and RT-PCR analysis, respectively. The expression of co-stimulatory molecules such as ICAM-1, B7-1, and B7-2 was also enhanced by CME. Furthermore, the activation of the nuclear transcription factor, NF-kappaB in macrophages was stimulated by CME.
CONCLUSION
Based on these observations, CME increased proinflammatory cytokines through the activation of NF-kappaB, further suggesting that CME may prove useful as an immune-enhancing agent in the treatment of immunological disease.
MeSH Terms
-
Blotting, Western
Cordyceps
Cytokines
Immune System Diseases
Intercellular Adhesion Molecule-1
Interleukin-6
Macrophages
Medicine, Traditional
NF-kappa B
RNA, Messenger
Transcription Factors
Tumor Necrosis Factor-alpha
Cytokines
Intercellular Adhesion Molecule-1
Interleukin-6
NF-kappa B
RNA, Messenger
Transcription Factors
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Cordyceps militaris Enhances MHC-restricted Antigen Presentation via the Induced Expression of MHC Molecules and Production of Cytokines
Seulmee Shin, Yoonhee Park, Seulah Kim, Hee-Eun Oh, Young-Wook Ko, Shinha Han, Seungjeong Lee, Chong-Kil Lee, Kyunghae Cho, Kyungjae Kim
Immune Netw. 2010;10(4):135-143. doi: 10.4110/in.2010.10.4.135.
Reference
-
1. Gai G, Jin S, Wang B, Li Y, Li C. The efficacy of Cordyceps militaris capsules in treatment of chronic bronchitis in comparison with Jinshuibao capsules. Chin J New Drugs. 2004. 13:169–171.2. Choi SB, Park CH, Choi MK, Jun DW, Park S. Improvement of insulin resistance and insulin secretion by water extracts of cordyceps militaris, phellinus linteus, and paecilomyces tenuipes in 90% pancreatectomized rats. Biosci Biotechnol Biochem. 2004. 68:2257–2264.
Article3. Yun Y, Han S, Lee S, Ko S, Lee C, Ha N, Kim K. Anti-diabetic effects of CCCA, CMESS, and cordycepin from Cordyceps militaris and the immune responses in streptozotocin-induced diabetic mice. Nat Prod Sci. 2003. 9:291–298.4. Yu R, Wang L, Zhang H, Zhou C, Zhao Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia. 2004. 75:662–666.
Article5. Cho MA, Lee DS, Kim MJ, Sung JM, Ham SS. Antimutagenicity and cytotoxicity of cordycepin isolated from Cordyceps rnilitaris. Food Sci Biotechnol. 2003. 12:472–475.6. Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol. 2005. 96:555–561.
Article7. Chen C, Luo S, Sun YJ, Zhang CK. Study on antioxidant activity of three Cordyceps sp. Chin J Biochem Pharm. 2004. 25:212–214.8. Ross JA, Auger MJ, Burke B, Lewis CE. Burke B, Lewis CE, editors. The biology of the macrophage. The macrophage. 2002. 2nd ed. Oxford, UK: Oxford Medical Publications;1–72.9. Kinne RW, Bräuer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000. 2:189–202.10. Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997. 100:2417–2423.
Article11. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001. 2:907–916.
Article12. Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005. 174:2825–2833.
Article13. Isomäki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997. 29:499–507.14. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002. 105:1135–1143.
Article15. Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992. 103:264–274.
Article16. Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998. 11:1218–1221.
Article17. Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001. 7:1291–1297.
Article18. Riehemann K, Behnke B, Schulze-Osthoff K. Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-kappaB. FEBS Lett. 1999. 442:89–94.
Article19. Renard P, Raes M. The proinflammatory transcription factor NFkappaB: a potential target for novel therapeutical strategies. Cell Biol Toxicol. 1999. 15:341–344.20. Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000. 6:441–448.21. Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989. 169:1543–1555.
Article22. Majumder N, Dey R, Mathur RK, Datta S, Maitra M, Ghosh S, Saha B, Majumdar S. An unusual pro-inflammatory role of interleukin-10 induced by arabinosylated lipoarabinomannan in murine peritoneal macrophages. Glycoconj J. 2006. 23:675–686.
Article23. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997. 15:323–350.
Article24. Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002. 20:825–852.
Article25. Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997. 240:419–424.
Article26. Kolb H, Kolb-Bachofen V. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol Today. 1998. 19:556–561.
Article27. Paterson RR. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008. 69:1469–1495.
Article28. Ahmad N, Chen LC, Gordon MA, Laskin JD, Laskin DL. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J Leukoc Biol. 2002. 71:1005–1011.29. Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994. 91:12013–12017.
Article30. Dazzi F, D'Andrea E, Biasi G, De Silvestro G, Gaidano G, Schena M, Tison T, Vianello F, Girolami A, Caligaris-Cappio F. Failure of B cells of chronic lymphocytic leukemia in presenting soluble and alloantigens. Clin Immunol Immunopathol. 1995. 75:26–32.
Article31. Deeths MJ, Mescher MF. ICAM-1 and B7-1 provide similar but distinct costimulation for CD8+ T cells, while CD4+ T cells are poorly costimulated by ICAM-1. Eur J Immunol. 1999. 29:45–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of Macrophages by the Components Produced from Cordyceps militaris
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- The Anti-inflammatory Effects of Water Extract from Cordyceps militaris in Murine Macrophage
- Anti-Inflammatory Effect of Mangostenone F in Lipopolysaccharide-Stimulated RAW264.7 Macrophages by Suppressing NF-kappaB and MAPK Activation
- Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages